Novavax has a good COVID-19 vaccine. How good is it? A very deep dive.
Let's separate the mythology from the data.
Novavax is the manufacturer of a protein-based COVID-19 vaccine (aka Nuvaxovid, or Nvx-CoV2373 for the ancestral variant vaccine) which exists as an alternative to the mRNA vaccines from Pfizer and Moderna. It’s a solid vaccine. Unfortunately, in some parts of the internet, people have alleged that there is a deep conspiracy of some sort to suppress Novavax from the public, that this vaccine is markedly better than mRNA vaccines in all respects, and anyone who chooses not to get it is making a grave error.
In an attempt to be comprehensive about the data and therefore fair to all interested parties, this is a longer post, so I totally get why people might not want to go through all of it. Here’s what you should know:
Available evidence consistently shows that the side effect profile with Novavax’s vaccine is milder than for the mRNA vaccines- this is a very good reason to take it if you’re someone who has a really hard time with the mRNA vaccines1. In fact, in my examination of the data for this vaccine, this is the ONLY compelling reason I could find to take it over mRNA.
Novavax produces an antibody response that is at least comparable to that of the Pfizer vaccine. This antibody response might be more durable than that of the Pfizer vaccine’s, but we need more data to say that with confidence.
Novavax clearly loses in a head-to-head comparison to mRNA vaccines (and even more so to adenovirus vaccines) when it comes to the CD8 (aka killer) T cell response. These cells are early responders in infection and are responsible for killing virally infected cells and are thought to be particularly for preventing severe disease (but less for infection/transmission). A major reason for this is that these T cells recognize parts of SARS-CoV-2 that do not undergo significant change with variants, but they cannot act until cells are already infected. Novavax has, however, shown solid protection against severe disease in clinical trials and in the limited real-world data we have despite this lackluster CD8 T cell response, but because such relatively small numbers of people have taken the vaccine, how it compares in protection is hard to say with confidence.
Data on Novavax as a booster to mRNA are mixed in terms of the relative quality of the immune response and the sample sizes of these investigations are small.
Novavax’s technology means that it cannot update to cover newest variants as quickly as mRNA can. Broadly speaking, it is okay not to perfectly match the circulating variant as the immune response generates breadth, but this does mean that there is a disadvantage relative to mRNA.
We have very limited data on how Novavax performs compared with mRNA in the real world outside of its initial pre-licensure studies, with the few studies we do have giving mixed results.
The pre-licensure trials indicate that Novavax’s COVID-19 vaccine, is, broadly speaking, safe; however, because of the limited number of doses of vaccine given around the world owing to Novavax’s challenges with production, we lack certainty about the risks of specific rare adverse events, i.e., myocarditis. With regard to myocarditis/pericarditis specifically, the risk is numerically slightly higher across the general population for Novavax than for mRNA vaccines, but it is unclear that this would hold as true if we had comparably large numbers of Novavax recipients. The risk for specific demographics known to be at higher risk for this adverse event (i.e., younger males) is not clear.
Contents so you can skip to the section(s) you care about
Pre-Licensure Trials and A Bit of History
Preamble providing context for Novavax as a company and where they were before the pandemic.
Preclinical development
Describes the early preclinical data on Novavax’s COVID-19 vaccine
Phase 1/2
Describes early human studies of Novavax’s COVID-19 vaccine
Phase 2/3
Describes efficacy studies of Novavax’s COVID-19 vaccine
Pediatric Data
Describes use of Novavax’s COVID-19 vaccine in kids
Real-World Effectiveness Data
Describes how Novavax’s COVID-19 vaccine has performed outside the context of clinical trials
Reactogenicity and Safety
Summarizes what is known about the side effect profile and potentially serious risks of Novavax’s COVID-19 vaccine
Novavax vs other COVID-19 vaccines: Primary series, Boosting, and Mixing
Contextualizes Novavax’s COVID-19 vaccine relative to other options, focusing on mRNA because of the data available
Design of Nuvaxovid
Detailed description of the composition of Novavax’s COVID-19 vaccine
Immunologic Basis of Nuvaxovid Action
Detailed description of how Novavax’s COVID-19 vaccine induces immune responses (intended mainly for those with a strong background in immunology)
Manufacturing Woes
Description of Novavax’s manufacturing issues that have limited commercial realization of their vaccine
Marketing Shenanigans and The Fandom
Directly addresses popular claims floating about Novavax’s COVID-19 vaccine and my personal issues with some claims from Novavax about how their vaccine works
The 2024-2025 Season COVID-19 Vaccine
Quick overview of relevant immunogenicity data from the JN.1-based Novavax vaccine.
Data Conclusions and Knowledge Gaps
Summarizes the knowns and unknowns of Novavax’s COVID-19 vaccine
Pre-Licensure Trials and A Bit of History
Before the COVID-19 pandemic, Novavax was struggling financially. It had not been able to get any vaccines to market to that point (to date its only licensed vaccines are for COVID-19 and malaria), and it was on the heels of a failed RSV vaccine candidate. Despite this, it had previously designed a protein nanoparticle vaccine against MERS-CoV, the coronavirus responsible for Middle Eastern Respiratory Syndrome, which showed protection in mice, and it was awarded $1.6 billion from Operation Warp Speed2. Basically: Novavax lacked experience as a pharmaceutical company, and that would prove to be a problem- but we’ll come back to that. Anyway, let’s look at the actual vaccine:
Preclinical Development
In August 2020, Novavax posted a preprint looking at their candidate vaccine, Nvx-CoV2373, in monkeys (cynomolgus macaques specifically), which was then published in November 2020. This study looked at how the monkeys responded to different doses of the vaccine, how they handled a challenge with SARS-CoV-2 based on their vaccination status, and benchmarked these responses against human convalescent serum. What was particularly impressive is that even though the macaques were challenged with a high dose of virus intranasally and intratracheally, viral subgenomic RNA (sgRNA; a marker of active replication) was undetectable in all vaccinated monkeys. The monkeys receiving the lowest dose of vaccine, however, did have detectable sgRNA in their lungs at day 2.
For reference, this is comparable to what was achieved in macaques with the Moderna vaccine (the dose of virus Moderna’s monkeys were challenged with was higher, however). The fact that virus was so well suppressed in the upper airway was a promising suggestion that the vaccine could suppress transmission of SARS-CoV-2. Unfortunately, as the adage goes: mice lie, monkeys exaggerate, and viruses evolve.
Phase 1/2
In September 2020, Novavax published Phase 1-2 trial data for their vaccine. This phase of the clinical trial is intended to help establish the safety of the product, including the appropriate dose. In the case of a vaccine, this is also used to gather data on the ability of the vaccine to induce an immune response (immunogenicity). A 2-dose series was tested looking at 2 doses of antigen (recombinant spike protein). Antibody responses were measured by comparing to those of people who had recovered from COVID-19. Impressively, the antibody levels induced by 2 doses of the vaccine were significantly higher than those of COVID-19 survivors (so long as spike protein was combined with the Matrix-M adjuvant):
One thing that should be noted here though is there is significant spread in the neutralizing antibody titers seen here. Additionally, the value reported here is an IC>99%, whereas IC50 (or reciprocal ID50 in this case) is a more standard metric. This complicates interpretations regarding how these responses compare to other vaccines (but we do have other studies available to help us out there- more on them later)3.
Phase 2/3
Novavax published two Phase 3 trials of Nuvaxovid. The first was published in June 2021 and took place in the UK., enrolling about 15,000 participants and randomizing them 1:1. The key results are below:
Some caution: A and C are time from the *second* dose. Efficacy after the first dose is nonzero and would be expected to appear from around 2-3 weeks after vaccination, and the second dose is given at 21 days after the first, so A and C *probably* do not demonstrate immortal time bias in their cumulative incidence functions. Broadly, there is very little doubt that this vaccine is effective. The measured efficacy in this trial was 89.7% (95% CI, 80.2 to 94.6) against symptomatic COVID-19, 86.3% (95% CI, 71.3 to 93.5) against the B.1.1.7 (alpha) variant and 96.4% (95% CI, 73.8 to 99.4) against non-B.1.1.7 strains, 83.4% (95% CI, 73.6 to 89.5) among all the participants starting 14 days after the first dose was, and 88.9% (95% CI, 12.8 to 98.6) overall effectiveness in those 65 and older.
In terms of safety, in general the vaccine was well-tolerated but there was a case of myocarditis after the second dose of vaccine in one recipient, which the DSMB judged to be likely viral myocarditis. One patient died of COVID in the vaccine group before he had the opportunity to receive a second dose of vaccine.
In an update, Novavax reported that in this study population over 7.5 months, efficacy against COVID-19 was 82.7% (95% CI 73.3 to 88.8) in the per-protocol population (meaning the people who got all the doses of vaccine as was to be done by the study protocol and didn’t withdraw) and 68.7 % (95% CI 58.1 to 76.6) in the intention-to-treat population (all participants in the study regardless of whether or not they followed the protocol):
For reference, past 4 months after the second dose, the efficacy of Pfizer was measured to be 83.7% (95% CI 74.7 to 89.9), and for Moderna it was 82.0 % (95% CI 79.5 to 84.2) at completion of the blinded phase which had a median follow up period of 5.3 months. I find it hard to make strong claims about the relative durability of Novavax to mRNA from these data.
PREVENT-19 also examined the durability of protection of Novavax’s vaccine up through the appearance of the Delta variant.
Per the discussion of the paper:
While comparison of the durability of the protective efficacy of vaccines is difficult to assess due to differences in study design, endpoints, strains, and duration of follow-up, qualitatively, NVX-CoV2373 aligns with the other vaccines with constant vaccine efficacy against pre-Delta strains through 3 months and waning against Delta over 5 months. Whether NVX-COV2373 has a durability profile similar to mRNA vaccines for later time points is unknown.
…
While our follow-up is shorter and sample size smaller compared with other vaccines, this analysis suggests that, for the period of follow-up studied here, NVX-COV2373 has a durability profile similar to the mRNA and vector-based vaccines for pre-Delta and Delta COVID-19. Heterologous boosting with different vaccine platforms may improve durability [2] and studies to evaluate heterologous boosting would provide useful data to inform vaccine recommendations.
In other words, there is no clear superior durability observed with Novavax’s vaccine against the Delta variant compared with other vaccines at the time.
In another Phase 3 trial in the US and Mexico published in December 2021, enrolling ~31,000 people, of whom ~30,000 underwent randomization (2:1, vaccine:placebo). Here are the cumulative incidence functions:
I personally would prefer it if the efficacy were reported for all outcomes with time starting from the first dose (this helps to confirm that there isn’t some kind of bias in the groups) but this is not disqualifying. Here’s a table of all the different efficacy outcomes:
In terms of safety, nothing obvious jumped out, but one case of myocarditis occurred in the vaccine group and one case also occurred in the placebo group; because this study was randomized 2:1, the incidence is actually numerically higher in the vaccine group in this study, but whether or not this is meaningful is anyone’s guess. The study did also institute a blinded crossover at 3-4 months, as withholding the vaccine from the placebo group after they had proven efficacious is not ethical. Notably, all data here is before the appearances of the Delta and Omicron variants.
Nuvaxovid may have had significant trouble with some variants, but it’s hard to say with certainty. For example, the beta variant (B.1.351) took root in South Africa, and was particularly alarming because it had substantial evasion of antibody responses elicited by two doses of vaccine or infection (more than any other variant before Omicron). Here, there is a drastic drop in the efficacy of Nuvaxovid, down to about 50%.
Notably, this population has a significant burden of HIV infection, which naturally can reduce protection from vaccines. However, when restricting the analysis to those who do not have HIV, the effect isn’t particularly dramatic with 60.1% (95% CI, 19.9 to 80.1) efficacy. Admittedly, at least part of this could be related to the limited follow-up time, as other vaccines initially also show poor effectiveness against the B.1.351 variant in particular before the second dose, and this study followed people only for 60 days. Nonetheless, I am not aware of Novavax ever explaining why this vaccine seemed to take such a hit in its efficacy when other vaccines seemed to fare much better.
For reference, Pfizer had reported that in its South Africa sites, 9 cases of COVID-19 occurred, all due to the beta variant, and all in the placebo group (for an efficacy of 100%, 95% CI, 53.5 to 100). Moderna did report data specific to the B.1.351 variant in its RCT data; however we do have real-world effectiveness data for the mRNA vaccines against this variant. Data from Qatar found 72.1% (95% CI, 66.4 to 76.8) effectiveness against symptomatic COVID-19 from the B.1.351 variant for Pfizer’s vaccine; in Israel, fully-vaccinated VE against confirmed or probable Beta infections was 72% (95% CI -5 – 97%; p=0·04) and against symptomatic confirmed or probable Beta infections was 100% (95% CI 19 – 100%; p=0·01) with no effectiveness for partial vaccination observed. For Moderna, data from Qatar found the vaccine to be 61.3% (95% CI: 56.5–65.5%) 14 or more days after the first dose but before receiving the second dose, and was 96.4% (95% CI: 91.9–98.7%) 14 or more days after the second dose against infection by the beta variant. Similar results were reported from France.
Pediatric Data
Novavax is approved down to age 12, supported by data from a Phase 3 trial in US adolescents. Adolescents were randomized 2:1 (vaccine:placebo) for 2 doses of Nuvaxovid. Because this was a later time point in the pandemic wherein infections were more widespread and variants had emerged, the study mainly sought to demonstrate that the vaccine induced similar responses in adolescents as it did in the adults where the vaccine had already proven protective. Still, safety and efficacy data were also collected:
Broadly, the vaccine was well-tolerated with no concerning differences between the vaccine and placebo groups in terms of adverse events. A more detailed look at specific adverse events is unrevealing of any concerning patterns, but this should be viewed in the context of the fact that this study enrolled ~2300 adolescents, and so rare adverse events are not detectable for a study this size.
The major variants during the study period were alpha and delta:
Most of the immunogenicity data is in the supplement; in general, responses are very good, although there is a surprisingly large drop in neutralizing titer with the alpha variant from ancestral. I also find it odd that the gamma variant has higher neutralizing titers than alpha. It is also worth noting that a substantial number of vaccinees lack detectable neutralizing antibodies to Omicron variants (BA.1, BA.2, BA.5), though these were not present during the analyzed study period.
Basically, the vaccine did the job it was intended to do here.
Another Phase 2/3 trial was performed with the vaccine produced by the Serum Institute of India (SII) known as Covovax (this is the same vaccine as Nuvaxovid just produced by SII) in Indian adolescents. Participants aged 2 years to 17 years were randomized 3:1 to SII-NVX-CoV2373 or placebo and monitored for 179 days. ~460 adolescents (12-17) and ~460 children (2-11) completed the study. Broadly, the study shows that the vaccine reliably induces antibody responses in vaccinees which are significantly greater than those in adults, and the responses in children are greater than those in the adolescents as well.
Real-World Effectiveness
There has been very limited real-world effectiveness data on Novavax because so relatively few people have taken this vaccine. We do have a study from Australia that attempted to compare the real world effectiveness of BNT162b2 (Pfizer), mRNA-1273 (Moderna), ChAdOx1 nCov-19 (Oxford/AZ) and NVX-CoV2373 (Novavax) against the Omicron variant:
In this study, Pfizer and Moderna both had the lowest incidence of infection, and Novavax in fact had a higher incidence than even Oxford/AZ for both the primary series and the booster, which is puzzling. Notably, this is specifically examining infection, and does not consider which of these individuals, if any, developed disease. Furthermore, within the booster cohort seen here, very few people received Novavax’s vaccine compared with the mRNA vaccines. This makes comparisons difficult, although numbers for the primary series cohort are similar enough that comparisons are likely reasonable. Still, we have seen that it takes at least 3 doses of vaccine to really get solid antibody responses against the Omicron variant, so those numbers are not as important. Nonetheless, this study fails to substantiate Novavax’s deified stature among COVID-19 vaccines.
In addition to this work, we do have two preprints examining the effectiveness of Novavax in Korea. This one examined 3 doses of Novavax compared with 3 doses of Pfizer, finding that there was a slightly higher risk of infection with Novavax and a numerically lower risk of severe infection that did not reach statistical significance (unfortunately, this is the highest resolution figure I could get from the preprint):
Within this data there is also a table which compares the risk by age groups. It appears that Pfizer’s advantage in this dataset is mainly in the 18-39 year-old age group, as for all other age demographics there is no statistically significant difference.
This study is however very limited in the confounders it was able to adjust for as these did not include things like socioeconomic status and comorbidities.
The second preprint we have also uses data from Korea, but it is perhaps worth noting that Novavax also has authors on this preprint. This does not in any way disqualify its findings, but it is worth bearing in mind. Here are the results:
Basically, Novavax shows a slight benefit over Pfizer against infections for a homologous primary series and first booster; comparisons for second boosters lack statistical power but Novavax’s vaccine appears to have a numerically lower risk, when looking over a period of 30 days after the last dose. In terms of severe infections, the incidence is numerically lower for Novavax but does not reach statistical significance in any case. There is an important detail here though. For every comparison group (primary series, first booster, second booster) Novavax’s recipients were significantly more likely to have had a prior infection, with the biggest difference in those who had the primary series only (77.5% vs 63.2%). This may be balanced in part by the fact that a greater proportion of Novavax’s recipients were likely to be disabled and have more comorbidities, but nonetheless, this complicates interpretations about how well the vaccines work relative to one another.
We also have one more effectiveness study using an Italian population from February 2022 to September 2022, when Omicron subvariants BA.2 and BA.5 were dominant. This effectiveness study has a bit of a different design than most, working by using the period from 3-10 days after the first dose as its reference interval. The thinking here is that this is before the vaccine would be expected to have any effectiveness, which is reasonable. The results are summarized below:
The study also excluded those with a documented SARS-CoV-2 infection, which helps. Broadly, the effectiveness of the regimen appears to be stable once the full vaccine series is completed over the 4-month period. However, because the design of this study differs in how vaccine effectiveness is measured, and there can be differences in how symptoms are reported across different studies, the comparison to other vaccines is difficult. In addition, measuring infections that are not medically attended (such as asymptomatic ones) is fundamentally very difficult because of the emergence of rapid antigen tests, and this could affect estimates in either direction if use of tests is not homogenous in the study population. Perhaps most importantly, this is based on a two-dose series of Nuvaxovid, so its meaning in a world where most people have had at least 3 doses is not entirely clear.
Another real-world study has also been published, which some are claiming substantiates claims about durability of Novavax being particularly good. The key figure is this one:
The problem here is this is not how you measure vaccine effectiveness. This is literally a plot examining how many people in your cohort didn’t get COVID-19. There is no comparison to unvaccinated people, or people who received a different vaccine. In addition, a very large portion of this study population includes individuals who have conditions considered by STIKO to be high-risk (54% had chronic neurological conditions alone), which may affect behaviors with regard to contracting COVID-19 to make them more cautious (hence the need for parallel comparison groups). It is also important to note that this study could not account for infection histories if infections occurred before 2022.
Reactogenicity and Safety
To start with, the pre-licensure trials reported reactogenicity and safety data::
Broadly, 2 doses of vaccine seem to be very well tolerated. Notably, the frequency of systemic symptoms is generally low and close to placebo, consistent with what is observed in the immunology of the vaccine (later section). It is noteworthy that the second dose seemed to be a bit tougher than the first, similar to the mRNA vaccines. For comparison, below are the abridged reactogenicity data from Moderna:
And from Pfizer:
In general, for the vast majority of people, the reactogenicity of mRNA vaccines is not nearly as bad as some people might lead you to think- reactogenicity is very frequent, but overall tends to be mild or moderate (i.e., does not significantly interfere with daily life). Despite this, a small percentage of a big number is still a very big number. This means that some people to experience disruptive systemic symptoms like fatigue, headache, muscle aches, joint pains, etc. to a degree that is disruptive. In general, these symptoms are short-lived (1-2 days or so) but nonetheless, can mean planning a vaccination is cumbersome. These symptoms are the result of an active immune response triggered by the vaccination and are not, in general, themselves harmful, though they may feel unpleasant. In a far, far smaller proportion of cases, the mRNA vaccines have been known to cause significant disruption in those who have certain chronic conditions, such as myalgic encephalomyelitis, aka chronic fatigue syndrome.
Still, because so many people have already gotten mRNA, the relevant question for most people is not “how does a primary series of Novavax look?” but rather “how does Novavax look as a booster to mRNA?” Here, Novavax has genuinely earned a gold star:
A literature review examining reactogenicity following mRNA boosters or Novavax in those whose primary series was mRNA vaccine found that systemic symptoms were significantly less frequent among Novavax recipients than those who received an mRNA booster, with the exception of nausea and vomiting for those who initially completed their series with Moderna’s vaccine. That one is a bit odd, and I can’t hazard an explanation. It could be a quirk of the relatively small sample sizes used.
Safety data on Novavax’s COVID-19 vaccine is a bit limited; it is true that it clearly met an acceptable measure of safety in its pre-licensure trials, which supports that the vaccine should be safe for the majority of people, but there are still some rare adverse events of interest. Myocarditis was a particular concern for Novavax’s vaccine before it was licensed because at this point it had been revealed as a serious adverse event following mRNA vaccination. Furthermore, Novavax actually had a numerically higher risk of myocarditis measured in its prelicensure trials than did either mRNA vaccine (see Table 20 for details). In general, this risk is very difficult to examine in the general population because (at least in the case of mRNA vaccines) it is strongly biased towards younger males, meaning that the increased risk in the general population could be negligible, but disproportionately affects them. Australia’s TGA has previously reported a rate of 3-4 cases of myocarditis per 100,000 recipients of Novavax compared with 1-2 cases per 100,000 doses of either mRNA vaccine, but they have also noted that the limited number of doses of Novavax mean there is reduced certainty in this estimate. CDC has also published that:
during a period in which 744,235 doses of Novavax COVID-19 vaccine were administered in Australia, Canada, the European Union, New Zealand, and South Korea, 35 reports (representing 36 adverse events) were identified among 20 male and 15 female vaccine recipients with a median age of 34 years (range = 23–62 years): 29 reports of pericarditis, including five in persons with a history of pericarditis after mRNA COVID-19 vaccine; four myocarditis cases; two myopericarditis cases; and one case of carditis, not otherwise specified. A postmarketing analysis from Australia identified three cases of myocarditis and 12 cases of pericarditis reported during a period in which 160,000 Novavax COVID-19 vaccine doses were administered (5).
CDC also published a safety report based on 69,227 doses of vaccine administered to people aged 12 and older; in general, there are no concerning or unusual patterns apparent here, but there is such a small number of doses that it is uninformative for considering rare adverse events. As of August 10, 2024, 83,047 doses of the vaccine have been given in the US per Our World in Data.
Novavax has published a risk-benefit analysis examining several scenarios for the vaccine’s effectiveness against the reported risks of their vaccine, finding that the balance of risks and benefits is still favorable; they do however note that there was a lack of detailed data for males aged 16-29, who represented the two cases that established the rate of myocarditis used in their clinical trials. Having said that, I do agree that assuming the course of myocarditis associated with Novavax’s vaccine is similar to that of the mRNA vaccines’, the balance of risks and benefits is favorable.
In addition to this, the EMA had issued warnings about the possibility of anaphylaxis, though a rate is not known and is likely very rare, and paraesthesias (pins and needles sensation in the skin) had also been reported.
Novavax vs other COVID-19 vaccines: Primary series, Boosting, and Mixing
Addendum 09/15/2024:
Recently this paper has come out comparing the viral loads for Novavax, Moderna, Johnson & Johnson/Janssen, and Oxford/AZ vaccines in the cases where infection occurred. Naturally, everyone wants to know which vaccine did the best, but there are a few important points to consider before we get that far (but if you’re in a hurry I can tell you that we can reasonably conclude that when it comes to the early variants of SARS-CoV-2, breakthrough infections with Novavax and Moderna are likely less effective spreaders of virus than those who are unvaccinated- beyond that, we cannot say much, including which vaccine did a better job).
Firstly, this is not viral load per se but PCR copy number. This is important to understand because while these quantities are related, they are not the same thing. In particular, when considering nidoviruses such as coronaviruses, subgenomic RNA (sgRNA) is produced in the course of replication, with genes at the 3’ end of the genome being present in greater copy numbers than those at the 5’ end:
This means that the choice of gene targeted by the RT-PCR can affect the copy number measured. For this reason, it is important that comparisons made be parallel. However, we encounter two issues on this front. The first is: it is not clear that the gene targets were harmonized across phase 3 trials, which makes direct comparison fraught. The second reason is there was significant variation in how swabs were collected across sites.
The paper works around this by limiting comparisons to the viral load corresponding to the nasopharyngeal swab closest to COVID-19 onset. Unfortunately… all 4 studies used different definitions of COVID-19 onset:
COVID-19 onset was defined as the date of first positive PCR test (AstraZeneca), symptom onset (Janssen), the earlier of the 2 (Novavax), or the later of the 2 (Moderna).
In addition to these, Moderna’s samples had to be limited to participants who were negative through day 57 by RT-PCR and anti-nucleocapsid serology assay, and who were PCR positive at diagnosis. Basically: I do not think a reasonable person can try to argue that the comparisons here are truly parallel. Nonetheless, the study is not without value. Here is the crucial figure:
The results are also summarized in tabular form:
First I think it’s important to address a point that many are perhaps not concentrating on but one that is still important. The difference in the “viral loads” for either adenovirus vaccine compared with placebo was not apparent (confidence intervals contain 0). This does not automatically mean that the vaccine does not reduce transmission. Remember- these are breakthrough infections. The vaccine reduces the likelihood of infection occurring in the first place.
I have already seen widespread proclamations that this proves the superiority of Novavax over Moderna in reducing viral load and therefore transmission. The issues with that claim are manifold. For one, there is the matter of basic statistics: Novavax’s estimates are much less precise than Moderna’s because they contribute far fewer samples to the analysis. This is manifested in a wider confidence interval. In fact, the confidence interval is so wide that it contains ALL of Moderna’s confidence interval and point estimates with it. The meaning here is that the difference in the viral load reductions are explainable by chance alone and do not necessarily reflect a true difference driven by a biological effect. It is also worth noting that in the distributions of viral loads, the placebo group for Novavax also tends to have lower viral loads than Moderna’s does (note the scale). This could reflect the relatively few samples, but it could also be an indicator that the comparisons are not parallel (again, note that the timing of the swabs relative to symptom onset is different). Additionally, if people want to compare the readout of viral load for effectiveness, we can’t ignore the other readout of effectiveness: the relative risk of a positive PCR result with Moderna vs. Novavax. For that we need to go to the supplement:
For Moderna, 594 cases were observed in the placebo group and 36 in the vaccine group (1:1 randomization). For Novavax, there were 60 in the placebo group and 11 in the vaccine group (with 1:2 randomization). This would correspond to a vaccine efficacy of 94% for Moderna and 72% for Novavax. Additionally, the paper writes:
There were no other statistically significant predictors of VL other than receipt of vaccine (Supplementary Figures 3–6). Time since last vaccination was weakly associated with VL only for mRNA-1273 (Spearman correlation = 0.30) (Supplementary Figure 7), although the estimated association was materially unchanged when this variable was adjusted (Supplementary Figure 8).
If we look in the supplement we see the following plots:
Indeed, there is a clear upward trend in viral load for Moderna for time since vaccination, and it is noteworthy that Moderna’s data are restricted to those on at least day 57 AND negative for nucleocapsid. For Novavax, it is less obvious, probably owing to the fact that there are just 11 data points to draw upon, but I do think a positive trend is also seen. The paper notes that the effect on viral load follows the basic trend of antibody titer- higher antibody titers result in greater viral load declines. It is possible that if we were to look before day 57, Moderna’s data might look a bit better as we might be closer to the antibody peak. Anyway, I don’t think strong conclusions can be drawn about which vaccin performs “best” given all of these limitations. In particular, given that this is based on variants that are, in pandemic time, ancient history, I don’t know how well the results translate to the modern context, which the authors also note. Let’s shift to more helpful studies to make comparisons, shall we?
One of the most valuable studies for understanding the different virtues and limitations of the different COVID-19 vaccines was published in 2022. This study was particularly valuable because it collected immune response data on four different vaccines (Pfizer, Moderna, Johnson & Johnson/Janssen, and Novavax) and SARS-CoV-2 infection at multiple matched time points and evaluated immune responses using the same assays, allowing for assurance that comparisons were parallel. Still, data on Novavax was a bit harder to get and more limited in terms of the time points taken, and fewer people who had Novavax were included overall:
Note that only 2 timepoints are taken for Novavax, with T4 being a bit later than the equivalent for the other vaccines, but T5 is matched. The basic results are summarized in the graphical abstract, although I do recommend checking back with the paper to see the details:
In terms of antibody responses (IgG) in the blood, Moderna and Pfizer were the winners with Novavax a close second, and infection lagging behind. The ability of these antibodies to neutralize virus was also greatest for Moderna, but Pfizer and Novavax were similar. The mRNA vaccines also won on memory B cell levels, which are important for the generation of new antibodies that cover new variants. In terms of T cells, Novavax was similar to the mRNAs in terms of the helper T cell responses, including the cTFH cells, a population thought to be critical for supporting antibody production. CD8 T cells, which kill virally infected cells and are early responders to new variants, were rare with Novavax, but induced at comparable frequencies with mRNA vaccines and Janssen; however, Janssen was unique in that CD8 T cell levels continued to rise throughout the entire study period. In sum, Novavax showed:
Comparable antibody responses to Pfizer, slightly lower than Moderna at ~6 months after the second dose; we cannot say much about the relative durability of antibody responses here because we only have 2 time points for Novavax
Comparable CD4 T cell responses to both mRNA vaccines
Significantly lower CD8 responses than either mRNA vaccine or Janssen (the graphical abstract is not clear on this point- check the original paper)
In short, a solid vaccine, but not obviously better in providing protection than the mRNA vaccines from looking at the immune responses over a period of 6 months after the first dose. One key limitation here is that immune responses at the upper respiratory tract were not measured for vaccines nor infection, which misses a big part of the picture. This group recently provided detailed characterization of the immune microenvironment in the upper respiratory tract.
The data for Novavax’s vaccine as a booster or mixed primary series is a bit all over the place. There is a general consensus that it a dose of Novavax on top of prior immunity from other vaccines does work to boost the immune response, so I won’t go through all of those studies because the result is expected and unsurprising. I think it’s more useful to consider how it works as a booster relative to the alternatives.
COV-BOOST looked at how well the vaccine worked as a booster in those who had other vaccines. In this dataset, those who received a Pfizer vaccine had better antibody responses if they were boosted with Oxford/AZ’s vaccine than with Novavax. Those who received an Oxford/AZ series first had a better response to Novavax than a third dose of Oxford/AZ. Among those who received Pfizer, the biggest boost response occurred in those who got Moderna.
T cell responses were also better if Pfizer was boosted with Oxford/AZ than with Novavax.
Com-COV studies the effects of mixed vaccine series and published several studies. Com-CoV2 randomized participants in the UK to receive the same vaccine as their first dose or a different one, and then compared the immune responses. Here’s the key data:
For those who received the Pfizer vaccine to start with, Novavax shows no clear advantage over either Pfizer or Moderna in antibody responses, and looks to be a bit worse in terms T cell responses.
In an update to Com-COV2, the durability of the immune responses was compared. Here, again, those who got Pfizer seem to be better off getting another Pfizer for their second dose, while those who got Oxford/AZ for their first dose seem to be better off with Novavax.
If we look at the fold change in antibody responses over time however, there is a bit of an advantage in neutralizing antibody responses from a Pfizer/Novavax series compared with a Pfizer/Pfizer series. At the same time, Oxford/AZ has the slowest waning when it’s used as a homologous series.
Com-COV3 looked at boosting adolescents who had a primary series of Pfizer vaccine with 2 different doses of Pfizer or Novavax. In terms of the antibody responses, Novavax has a bit of an advantage over Pfizer in the adolescents:
The study also tracked the frequency with which each group developed COVID-19 (A = all participants, B = participants who haven’t had COVID before, C = participants who had COVID before):
Viewed in aggregate, Novavax does have an advantage over either dose of Pfizer, though it is slight for the higher dose. However, the trends differ with those who had COVID-19 before and those who did not. In those who had COVID-19 before, mRNA fares better at either dose. In those who haven’t Novavax is superior. It should be noted that the size of these groups is quite small, including the seropositive group (C), so caution should be exercised against strong conclusions about how well the vaccine works; in terms of self-reported infections across all adolescents, the adjusted odds ratio for Novavax vs. high-dose Pfizer was 0.34 (95% CI 0.09-1.20) for 132 days post-second dose, and 0.65 (95% CI 0.27-1.54) for 236 days post-second dose per supplementary figure 7.
Data from South Africa looking at a third dose of Novavax’s vaccine support that it was able to induce neutralizing antibody responses against Omicron comparable to that of a third dose of Pfizer:
It should however be noted that the Pfizer vaccine antibody titers had more time to wane and Pfizer vaccinees were on average significantly older than Novavax vaccinees in this study.
In another study, healthcare workers who received an mRNA vaccine booster (third dose) 12 weeks prior and had confirmed no prior history of COVID-19 were invited to receive either Novavax or mRNA as a booster. 101 received Novavax, 59 received mRNA. Here, not only did Novavax give significantly worse antibody responses for every variant:
as well as worse T cell responses:
but the study actually tracked how many of them went on to get COVID-19:
and those who got Novavax had significantly more (40.6% vs. 22.0%) at day 84 post-boost. A key limitation here is that participants were not randomized- they were able to choose which vaccine they wanted to get. Novavax might have been favored by those who had a hard time in terms of reactogenicity with mRNA vaccines. The discussion section also notes that it is likely that more of the Novavax recipients were nurses, who have particularly high risk of exposure, possibly skewing the results. Nonetheless, the immune response data suggest Novavax to be inferior as a booster to mRNA than mRNA, which agrees with the incidence data. Otherwise, the vaccine was well-tolerated.
In sum, as a booster to mRNA vaccines, Novavax doesn’t seem to be better in most groups than mRNA as far as immune response or protection, but the data we have to draw upon here is limited. It is better tolerated in terms of reactogenicity, though.
Design of Nuvaxovid
A comprehensive description of the Novavax COVID-19 vaccine’s composition can be found in its patent; what follows is my summary and commentary.
Nuvaxovid is made from recombinant spike protein nanoparticles which have undergone prefusion stabilization with a double proline substitution (just like mRNA and Johnson & Johnson/Janssen’s vaccine), deletion of the polybasic cleavage site (aka furin cleavage site or FCS; neither mRNA vaccine did this but like Johnson & Johnson/Janssen’s vaccine did), known as the 3Q-2P-FL spike protein and is expressed using a baculovirus vector in Sf9 cells. Sf9 cells are insect cells from the fall armyworm (Spodoptera frugiperda).

It has been argued that deletion of the polybasic site is an important element of the immunogenicity of Nuvaxovid. In brief, when SARS-CoV-2’s spike protein is produced, it undergoes pre-processing wherein host proteases cleave the spike protein at the FCS, which separates it into S1 and S2:
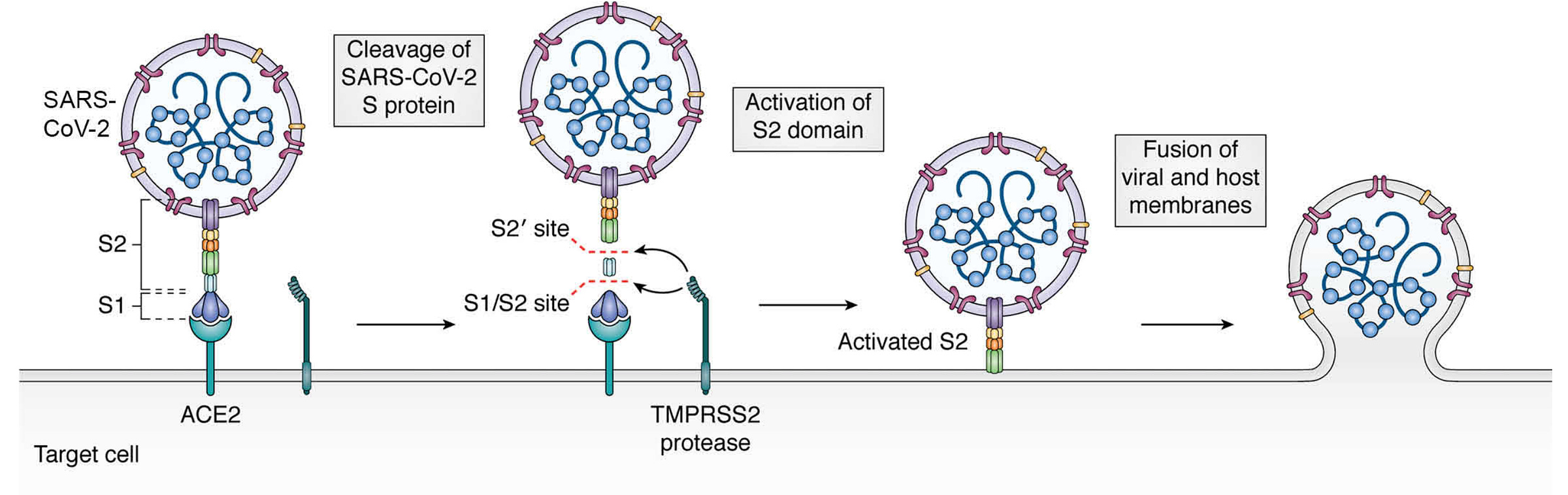
S1 contains the receptor-binding domain required for the virus to engage with its receptor and enter cells, so while cleaving it may sound like a bad idea, S1 remains in place by noncovalent interactions and typically will not dissociate until ACE2 is bound. Nonetheless, S1 can sometimes spontaneously dissociate from the spike protein, taking with it a bunch of important targets for antibodies4. Importantly, these would not be lost altogether, as S1 would still be secreted and can be targeted by the immune system, but this means loss of higher-order displays of S1 that can help with the immune response. Animal models have suggested that protection induced by proteins bearing both deletion of the cleavage site and the double proline stabilization give superior protection to proteins that only have one or the other, but how this holds for proteins made within our own bodies (as happens with mRNA vaccines or viral vectored vaccines is not known); in particular it should be noted that a major consequence of the double proline substitution is also markedly enhanced expression of the spike protein, meaning that even if removal of the cleavage site has a benefit when doses of protein are equivalent, this does not automatically mean that the advantage is significant when the protein is made by our own cells. I was unable to find data examining how the immunogenicity of full-length spike protein encoding mRNA vaccines differs based on the presence/absence of the polybasic site, which is curious.
Once these spike proteins are purified from the Sf9 cells, they are arranged around a nanoparticle core using polysorbate-80 micelles, and then adjuvanted with QS-21, an extract from Quillaja Saponaria (QS; the soap bark tree) used as part of the Matrix-M adjuvant proprietary to Novavax (42.5 micrograms of Fraction A, 7.5 micrograms of Fraction C per dose of Nuvaxovid). The other part of Matrix-M includes phospholipids and cholesterol to reduce the toxicity of the QS-21 saponins, which normally cause hemolysis and immediate pain at the injection site if not co-formulated in this way.
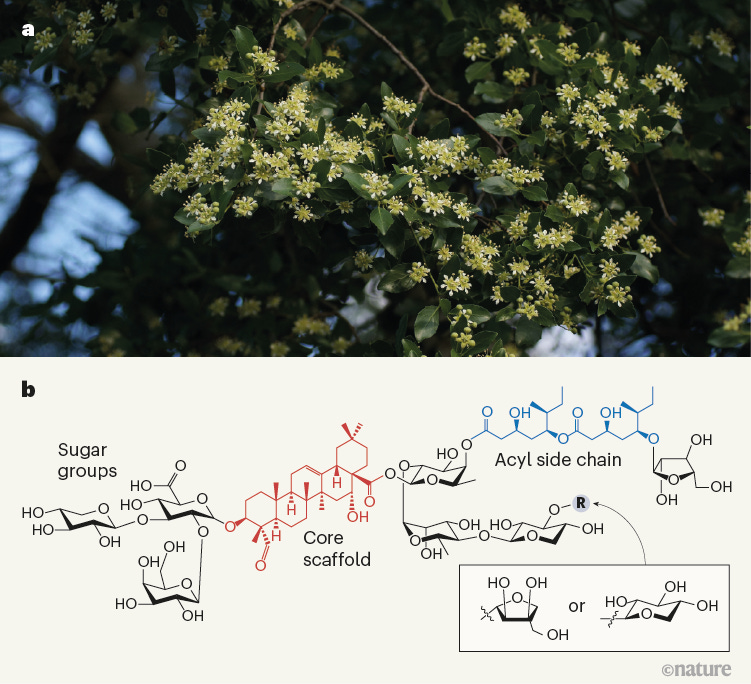
Matrix-M is part of a class of adjuvants known as immune stimulating complexes (ISCOMs) initially designed to make viral glycoproteins more immunogenic. Initially, the goal was that the proteins themselves would embed into the ISCOMs, but proteins had to be hydrophobic to achieve this, which required modifications in most cases, and that was a lot of work. Subsequent work showed that the proteins and the ISCOMs do not need to interact for the adjuvant effect of ISCOMs, meaning they could be present in their native form. Matrix-M does not appear to directly interact with the spike protein nanoparticles. Further detail on how Matrix-M contributes to the immune response is given in the Immunology of Nuvaxovid section.
The final product looks something like this:
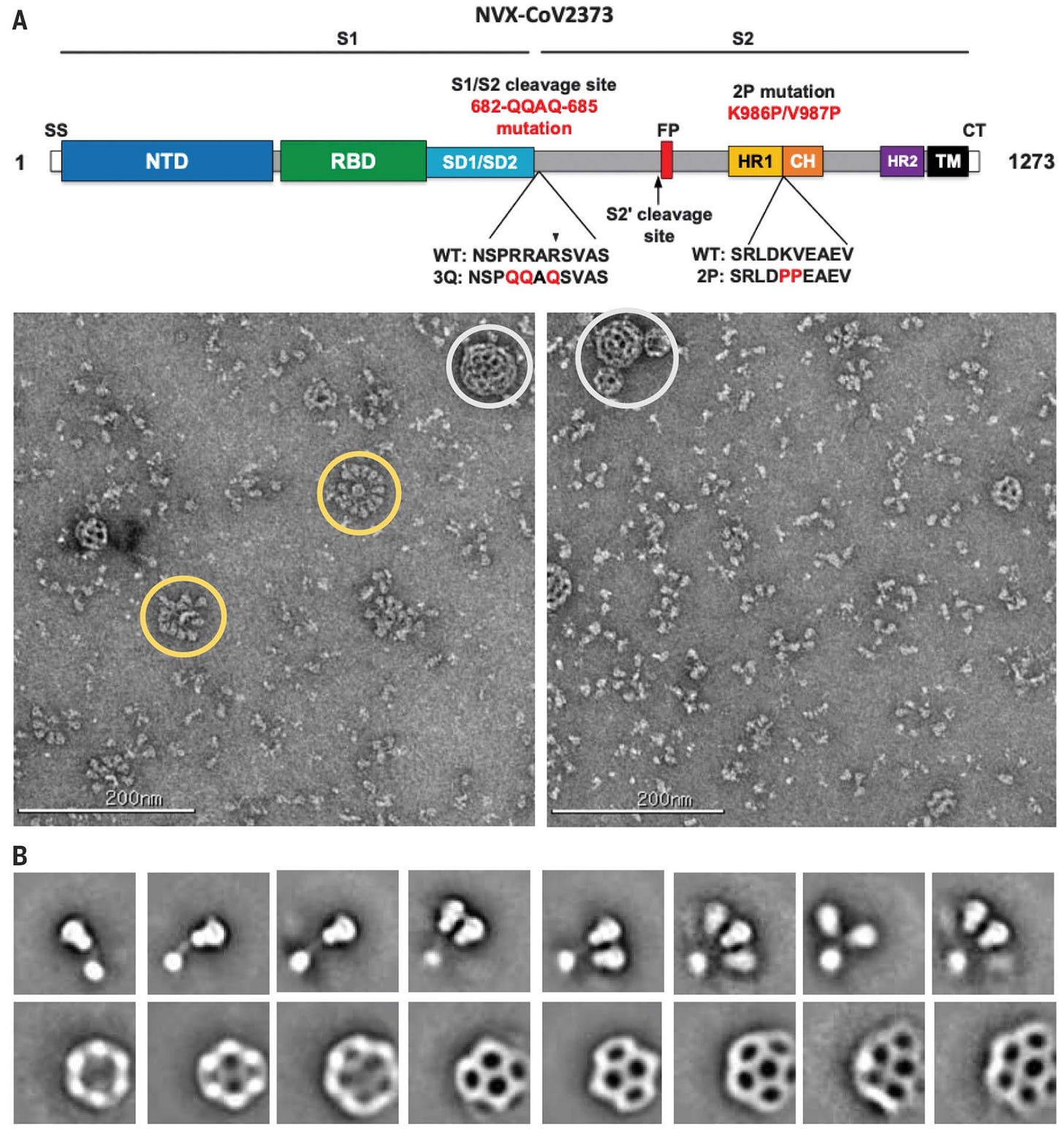
One thing that does bother me about this construct from the images shown above is that while it is advertised as a nanoparticle, bona fide repetitive displays of the spike protein trimers seem to be uncommon in the vaccine samples shown, with many of the spikes appearing as free trimers not associated with micelles. Furthermore, even though rosettes (yellow circles) can be seen, they seem to vary significantly in the number of spikes they have (up to 14 per micelle occur per this paper). This is not a safety issue, but, the close clustering of the spike proteins together in the orientation that would occur on the virus (i.e., with the receptor-binding domain facing out) is thought to be important in producing the advantages in the immune responses that occur with nanoparticle immunogens. I find it a bit strange that Novavax did not opt to chemically link spike proteins together after purifying them so that this could not happen, such as through sortagging to some scaffold or fusion to proteins that already spontaneously assemble into nanoparticles like lumazine synthase or ferritin, as this would theoretically result in much more uniform and consistent nanoparticle structures. I do also wonder a bit about the purification processes here as per the EMA’s assessment report, the product undergoes HPSEC-MALS (high-performance size-exclusion chromatography multi-angle light scattering) which should be able to isolate the spike proteins present in a nanoparticulate form, unless the rosettes are unstable and the micelles cannot reliably hold on to the spike proteins after isolation (which brings me back to my point of… why would you do it this way?). The EMA report does also note that a broad population of large particles is observed following this procedure. I do also note that the distribution of antibody titers for vaccine recipients seems to be wider than for other vaccines, which makes me wonder about how consistent doses are in their composition, particularly viewed in light of Novavax’s manufacturing issues.
The use of Sf9 cells to produce the spike protein is also thought to be important to the design of Nuvaxovid. As cells make proteins, part of the quality control mechanism for them involves the attachment of sugars (glycans) to specific sites on them, which is known as glycosylation.
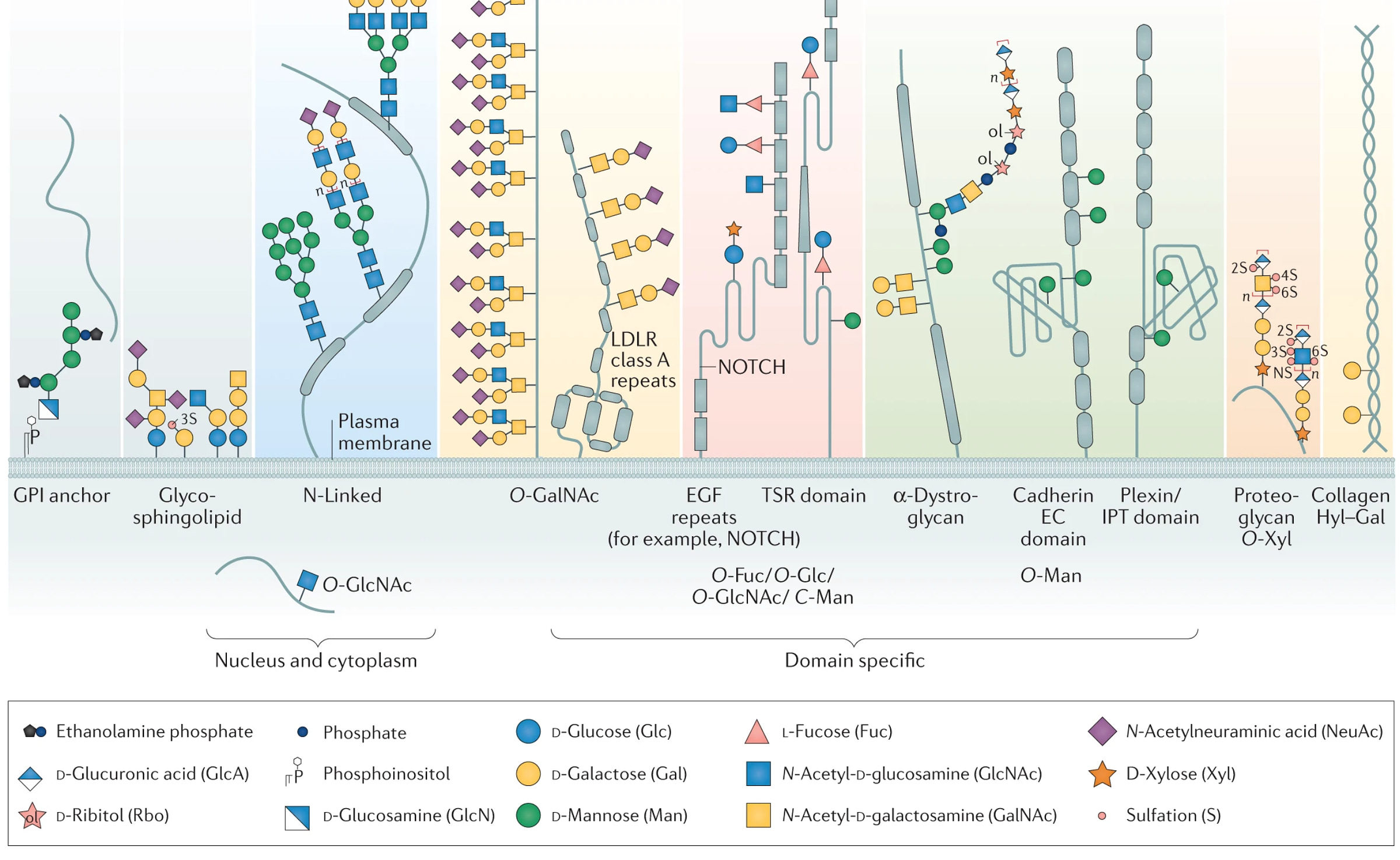
For some proteins, this glycosylation also has critical functional importance. How proteins get glycosylated is a function of the cell, as this determines the expression of glycosyltransferases (enzymes that attach or remove these sugars). In the context of the immune system, these glycans can pose an issue in immune responses because the immune system will not produce antibodies against glycans that originate from our own cells5. Viruses can exploit this to shield key sites on their proteins from antibodies (this is known as glycan shielding6):
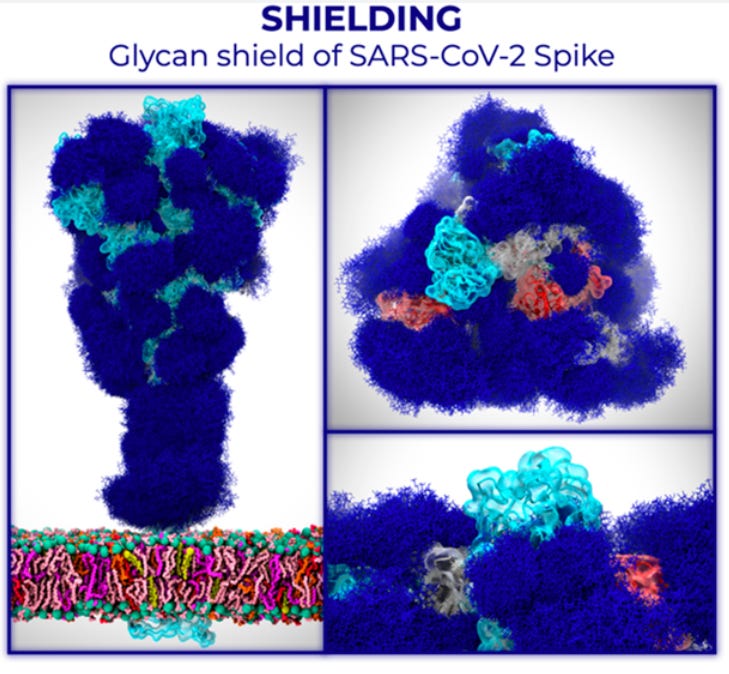
Insect cells, however, are interesting, because they produce glycans that are shorter and less complex than those that humans do:
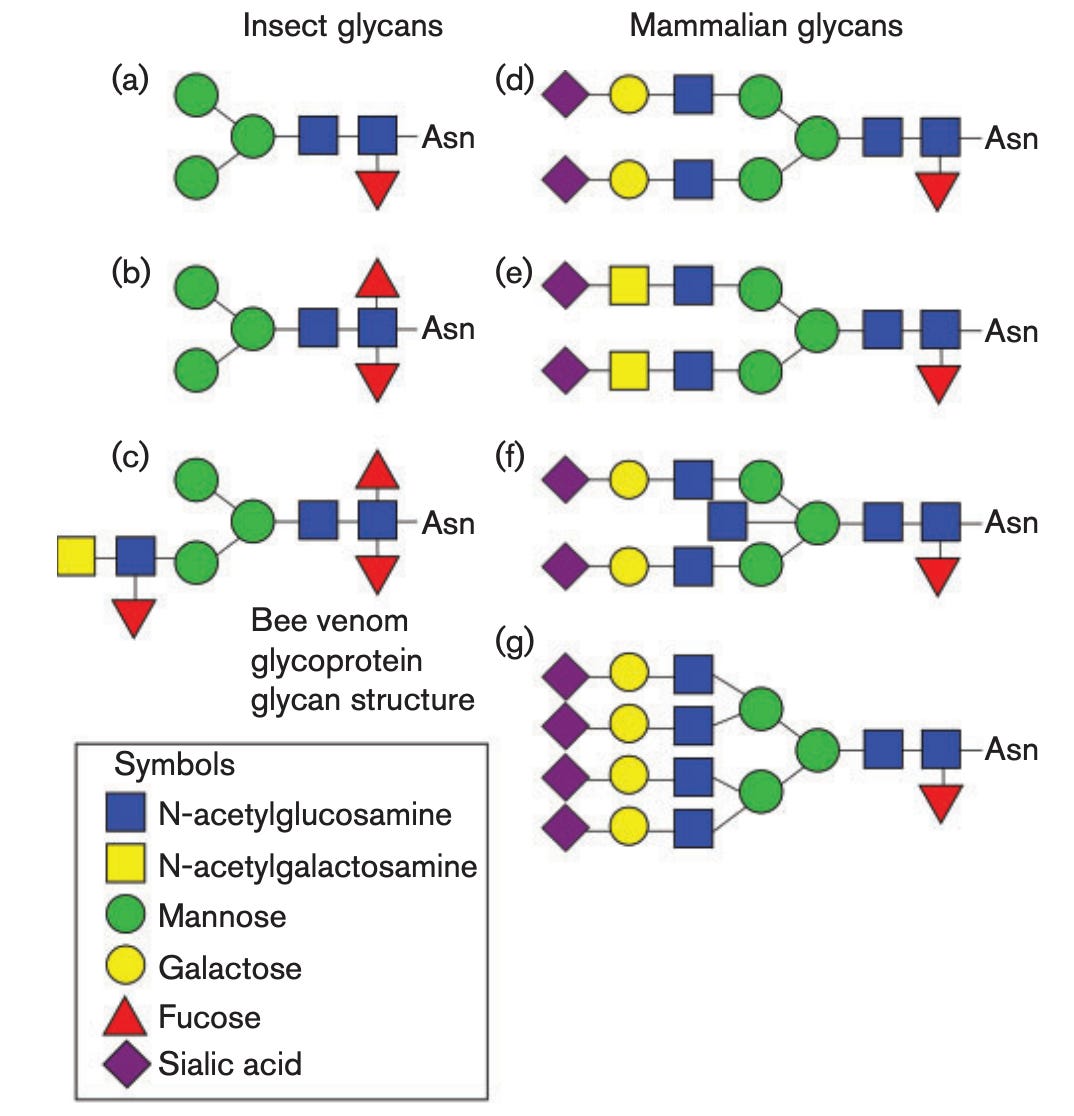
This means that insect-derived glycans make for much smaller glycan shields, which expose more of the protein epitopes. Some literature suggests that when glycosites (the parts of the protein to which glycans are attached) are removed from the spike protein or the glycans are trimmed by enzymes, this results in a broader antibody response, i.e., one that is more resilient to the changes that occur with variants. It is therefore conceivable that the use of insect cells to make this spike protein has a benefit for the quality of the immune response, although there is no head-to-head immunogenicity comparison I am aware of looking at the effect expression of this spike protein produced from Sf9 vs a mammalian cell line7.
Additionally, credit where credit is due, Nuvaxovid’s storage requirements are much simpler (standard refrigeration suffices) than those of mRNA vaccines.
Immunologic Basis of Nuvaxovid Action
The unique features of the immunogenicity of Nuvaxovid principally depend on the adjuvanticity of Matrix-M8, with a role for the spike protein modifications as well as described in the previous section. The mechanism of action of Matrix-M is complex, but the key component of the mixture appears to be the QS-21 saponin, although other saponins (QS-7, QS-17, and QS-18) likely contribute as well9. Nonetheless, these are not considered to be as potently immunostimulatory as is QS-21. QS-21 is a component of several adjuvants, including AS01, AS02, AS05, and AS15, and much of the mechanistic data on Matrix-M is therefore an indirect result of work studying the mechanism of AS0110. Nonetheless, it is believed that the mechanisms at play should be similar, and dissection of AS01’s adjuvant components (i.e. monophosphoryl lipid A (MPLA), a TLR4 agonist vs. QS-21) has been conducted. It is possible that now that a complete biosynthesis (here and here as well) of QS-21 has been achieved11, more detailed mechanistic insight into QS-21 based adjuvants may be possible, as the cost of even miniscule quantities of QS-21 is astronomical ($400,000 per gram) in part because of deforestation contributing to scarcity of the soap bark tree, its native botanical source, coupled with the very restrictive conditions in which the tree yields QS-21.
QS-21 is an amphiphilic triterpene glycoside which contains either an apiose (QS-21A) or xylose (QS-21X) moiety in a ~2:1 ratio. It is unstable under alkaline conditions but can be incorporated into liposomes (40 nm in size in the case of Matrix adjuvants) with phospholipids and cholesterol to both reduce its toxicity/reactogenicity and retain its immunopotentiating properties; when used independently of these, its toxicity is considered to be prohibitive for use in humans. The immunostimulatory consequences of QS-21 are well-defined, but the mechanisms by which they are achieved are more poorly understood12. These are summarized in the figure below from Novavax:
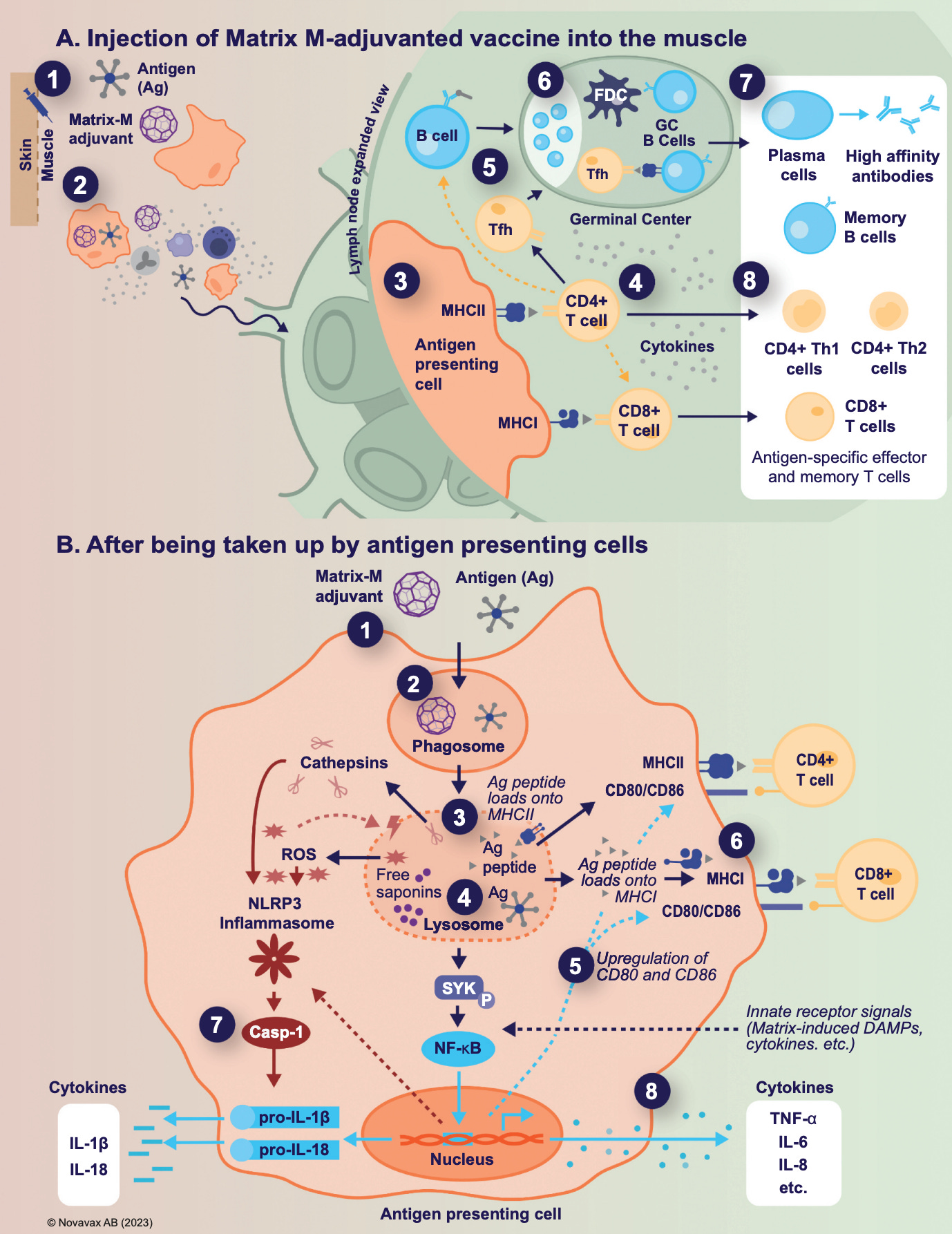
Upon administration of Matrix-M, antigen-presenting cells take up the adjuvant (as well as antigen), and become activated. This leads to their migration into lymph nodes and production of key cytokines and chemokines (interferon-γ [IFN-γ], interleukin [IL]-1β, IL-6, tumor necrosis factor [TNF]-α, CXCL1, CCL2, CXCL9, and CXCL10). One of the major effects of this adjuvant is a dramatic (but brief) increase in the cellularity of the lymph nodes, the fold-change of which is greatest for neutrophils, but also recruits B cells and T cells, which can result in lymphadenopathy13. QS-21 has been shown to induce a Th1-based response, as well as promote the induction of TFH cells. In addition to these, it is believed that QS-21 can induce pores in the endosomes of antigen-presenting cells that permit cross-presentation to occur, allowing for induction of CD8 T cell responses. Nonetheless, while some induction does occur, this is modest for Nuvaxovid14.
QS-21’s has been proposed to cause inflammasome activation, a mechanism it shares with several other adjuvants, although it is suggested that this mechanism is not important for its in vivo adjuvanticity. Nonetheless, inflammasome activation induces proteolytic activation of IL-1β, which is released through pores in the cells taking up QS-21. Upon formulation in liposomes, QS-21 disrupts the structure of lysosomes, causing release of proteases into the cytosol that trigger activation of spleen tyrosine kinase (Syk), which induces NF-κB activation that triggers the production of cytokines such as IL-6, IL-8, and TNF-α. Owing to its carbohydrate moieties, QS-21 has been suspected to engage with lectins as a mechanism for its adjuvanticity, which helps to permit uptake into antigen-presenting cells15. In addition to this, it has been proposed that QS-21’s aldehyde can form imines with the ε-amino group of lysines present on T cells, and in doing this to the CD2 receptor, can provide costimulation to T cells. The aldehyde group is believed to be essential for Th1 polarization of the immune response, as it does not occur with QS-21 derivatives lacking the aldehyde. Human monocytes treated with AS01 demonstrate substantially increased expression of CD86 and CD11c; AS01 demonstrates additional enhancement of HLA-DR expression. QS-21 has also been shown to co-localize with CD169+ resident macrophages in the draining lymph nodes, wherein it appears to cause loss of lymph node macrophages that ordinarily capture lymph-borne particles in a manner similar to lymph borne infections. The effect is greater for subcapsular sinus macrophages than medullary macrophages16. If these macrophages are depleted prior to immunization with clodronate liposomes, QS-21’s adjuvant action appears forfeit, implicating these cells as being critical mediators of adjuvanticity. HMGB1, released through inflammasome pores together with IL-1β and IL-18, appears to be important for inducing T cell polyfunctionality through engagement of TLR4; however, this raises questions about the additional benefit of MPLA inclusion in AS01, which does not appear to be redundant in its adjuvant behavior based on other data.
Detailed immunogenicity profiling of Nuvaxovid has been conducted in nonhuman primates, though there should be some caution as findings may not perfectly translate for humans. Broadly, data support that Matrix-M facilitates a highly localized immune response in draining lymph nodes from the injection site, with declines in T lymphocytes on days 1 and 14 following vaccination coincident with the expression of the lymph node homing receptor CCR7; NK cells and B cells also decline in serum but expression of CCR7 is not significantly changed, suggesting that homing to lymph nodes may be facilitated by cytokines other than CCL19 or CCL21. Over the 14 days following immunization, the most salient increases in plasma cytokines occur for SDF-1α (stromal cell-derived factor 1α or CXCL12), IL-8 (CXCL8), CCL11, and CCL4, supporting the highly localized nature of the immune response. There is also a progressive increase in CD40L in serum over the 2 weeks following immunization. In addition, there is successive diversification of the antibody response against the spike protein with subsequent vaccination, initially concentrated on RBD epitopes but then shifting outside this. Most likely, this is a consequence of epitope masking and antibody feedback. Notably, the immunodominance hierarchy in the macaques is a bit different from humans receiving mRNA, as the first dose of vaccination principally elicits RBD-targeting antibodies in the macaques, whereas in humans receiving mRNA vaccines, S2 epitopes are the principal targets. The authors reasonably suggest that this may reflect the immune history of prior betacoronavirus infections in humans and note that follow-up in a human cohort of Novavax vaccinees is warranted. With regard to antibody avidity, the largest increase occurs with the administration of the second dose of vaccine, rather than the third. There is progressive accumulation of plasma cells in the bone marrow of rhesus macaques, increasing with successive doses of vaccine, with peaks occurring near the time of vaccination and contraction thereafter; however, though the bone marrow does house long-lived plasma cells, most plasma cells in the bone marrow are not long-lived, meaning that, while this is encouraging as a comment on the durability of humoral immunity, it is not definitive. IgG did appear in the lungs as well in proportion to serum IgG titers, but IgA content in the BAL fluid was too low to be detectable. The antibodies themselves demonstrate strong ability to induce antibody-dependent cellular phagocytosis (ADCP), antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent complement deposition (ADCD). However, ADNP declines substantially when considering the Omicron spike protein for rhesus macaques vaccinated with ancestral and P.1 spikes, though the other effector functions are preserved; the basis for this is unclear. Interestingly, a substantial proportion of the serum antibody response appears to include IgM. In terms of T cell responses, memory Th1 cells responsive to spike are present in the lungs and blood following immunization; cTFH cells are also present in the blood but are not appreciably detectable in lungs. Memory Th1 cell proportions decline with time from vaccination but are restored with booster vaccination. CD8 T cell responses in the macaques are minimal, which is also seen in humans. Though this detailed immunogenicity profiling is very valuable in understanding the functional consequences of immunization with Nvx-CoV2373, lack of parallel comparison to other COVID-19 vaccines limits interpretability.
In addition to this work on non-human primates, a study examining Novavax’s vaccine as a heterologous booster to mRNA-1273 has been published. As with humans, late after the second dose of mRNA, a prominent class switch to IgG4 was observed; curiously, the proportion of spike antibody that was IgG4 was greatest for those macaques receiving Novavax’s vaccine, and particularly for those receiving Nvx-CoV2515, a BA.5 variant vaccine. Furthermore, IgG antibodies in secretions following this booster are slightly lower than those receiving homologous mRNA-1273, but the differences do not reach statistical significance. In terms of variant-specific neutralization, all boosters produced largely similar titers in the macaques, though a numerical advantage was apparent for all variants except BA.2.75.2 for mRNA-1273 (in that case, Nvx-CoV2515 had the edge). Titers also declined at a similar rate independent of the booster vaccine. 3 months after vaccination, macaques were challenged with 6 × 105 PFUs of the SARS-CoV-2 BA.5 variant administered as 2 mL by the intratracheal route and 1 mL by the intranasal route. All vaccination regimens effectively suppressed the appearance of viral RNA in the BAL fluid, though a single macaque in the mRNA-1273 homologous boost group did have transient appearance of subgenomic RNA on days 2 and 4 at levels higher than macaques receiving Nvx-CoV2373 or Nvx-CoV2515. Nasal subgenomic viral RNA proved much more difficult to suppress, with significant heterogeneity across macaques, but numerically higher viral loads among those receiving homologous boosters of mRNA-1273. Numerically, Novavax-boosted primates had a slight advantage in RNA count, though, curiously, this was greatest for Nvx-CoV2373. Serum BA.5 neutralizing antibody titer was the strongest negative predictor of nasal viral subgenomic RNA. Nonetheless, given that each group had just 6 macaques, strong conclusions about the relative protection of heterologous vaccination regimens in humans seem unwarranted, particularly given the existence of human-specific data on this question described earlier.
Manufacturing Woes
One of the issues that at times gets glossed over in discussing Novavax, which is a very substantial issue, is the significant manufacturing problems the company has experienced. This is a major reason Novavax was so delayed in getting to market. Because of the failure of their RSV vaccine in 2016, Novavax had to lay off 30% of their personnel and sold their plant in 2019, leaving them with no internal manufacturing facilities. Supported by Operation Warp Speed’s $1.6 billion and $384 million from the Coalition for Epidemic Preparedness Innovations (CEPI), they partnered with Contract manufacturing organizations (CMOs) and members of the Developing Countries Vaccine Manufacturers Network (DCVMN). In the US, Emergent BioSolutions was spearheading manufacturing of the vaccine, which would prove to be a problem as the company had major problems manufacturing the adenovirus vectored vaccines by Oxford/Astra Zeneca and Johnson &Johnson/Janssen vaccines. Eventually, Novavax’s production in the US was displaced when the facility was designated for the manufacturing of other vaccines. Eventually, the supply chain for Novavax became extremely convoluted. As King writes:
The drug substance for the European supply chain was expected to come from the Bohumil plant in the Czech Republic (which they purchased) and Biofabri in Spain. The US supply was expected to come from the CMO FujiFilm in both North Carolina and Texas. The Asian supply was expected to come from SK Biosciences in Korea, the Indian supply from Serum Institute of India, and the Japanese supply from Takeda [24]. The technical transfer of the drug substance process into so many diverse factories was very challenging and did not go as expected [37], [38]. Significant issues developed with yields and inconsistent purity of the drug substance, which varied in purity between the various facilities. These manufacturing problems may have contributed to delays to regulatory authorizations [38], [39], [40]. The WHO and EMA EUL was obtained on December 20, 2021 based upon the CMC data from the manufacturing process used at the Serum Institute of India.
The FDA-approved version of vaccine was produced in India by the Serum Institute of India, which was a first in the history of the FDA. The EMA soon approved it shortly after the FDA using the vaccine produced by SK Biosciences in Korea.
I’ve observed some people try to behave as though manufacturing problems on the part of Novavax are minor concerns given that they have such a good product. This is an extremely naive perspective. Fundamentally, if your vaccine exists only in theory, if you cannot actually make the vaccine you claim to make, your vaccine does not actually exist. Novavax’s Texas plant for example failed to produce vaccines meeting purity standards (70% when at least 90% is expected). Purity of vaccine products is critical because, being made in cells (with the exception of certain mRNA vaccines), there can be exposure to any number of adventitious agents from those cells whose effect on human health is not characterized. In principle, these are less of an issue for subunit vaccines such as Novavax’s, and yet the manufacturing capabilities of the company’s CMOs ran into multiple roadblocks. Based on the delayed time to market with even recent iterations, scalability still remains incompletely resolved. More recently, Novavax has reported that it will contract with Sanofi for its manufacturing, which should hopefully improve the situation as Sanofi has extensive commercial success and experience with vaccines. Nonetheless, approval of their latest vaccine, a SARS-CoV-2 JN.1 spike protein-based one, has been delayed. Novavax did report that they expected to be ready by September 1 at the VRBPAC meeting, so long as it was a JN.1 vaccine, and indeed, on August 29, 2024, the FDA granted approval for this vaccine.
Marketing Shenanigans and The Fandom
It is difficult to disentangle Novavax’s claims from the claims of “The Fandom,” that is, the stalwart devotees of Novavax who have taken the data available on this vaccine and stretched the truth about it well past the breaking point17. Nonetheless, Novavax has presented their vaccine at times in ways that are, I think, deceptive, and there is one particular instance I find morally reprehensible. It is of course possible that there is a genuine ignorance on their part regarding why their comments are incorrect or misleading, but I don’t know that that’s any better given that they are a manufacturer of a vaccine and should have extensive subject matter expertise therein.
First, the stuff that’s purely fan fiction:
There is essentially no good evidence that Novavax gives a much better antibody response than does mRNA. I hope I’ve detailed why above, but for an explicit demonstration, below is a slide from the June 2023 VRBPAC meeting concerning strain selection for the 2023-2024 season made by Novavax:
If you see markedly better neutralization here for Novavax than for mRNA, I suggest you get your eyes checked. Sure, maybe titers for the ancestral spike protein in 3 Novavax are higher than for 3 mRNA, but given that this version of spike is basically totally out of circulation, I’m going to say irrelevant. In every other comparison, the differences are pretty minor.
Durability of protection from Novavax’s vaccine is not clearly much better than mRNA. Novavax themselves says their durability is comparable to that of mRNA (read: not clearly better).
Novavax’s vaccine has seen limited real-world use (again, because they have had serious manufacturing problems), which complicates attempts to assess its performance. In situations like this in vaccinology, we are greatly aided by correlates of protection. A correlate of protection (CoP) is some aspect of the immune response that can be measured which predicts (at the population level, at least) protection from some outcome of the disease the vaccine protects against. In general, this is going to be an antibody response, in large part because the field has extensive experience with evaluating antibody responses, but this doesn’t necessarily mean that antibodies themselves are responsible for protection.
This has been done for the efficacy trials of the vaccines and antibody levels that correspond to various protection levels have been established (formally, in Plotkinian nomenclature, this is known as an absolute correlate, but within the field usage is sloppy, and people tend to use “correlate of protection” when they mean “absolute correlate”)- but there’s a problem here. These data are all based on the virus in the form it was present in during the trials, meaning: pre-Omicron, pre-Delta, etc. Since the emergence of these variants, SARS-CoV-2 has shifted in what part of the body it infects and how quickly (Omicron for example has a much greater affinity for the upper respiratory tract than older variants, which maybe reduces the severity of disease, though not nearly as much as some would like, but also makes it MUCH harder to control the spread). This means that you cannot just measure an antibody response to the variant you’re interested in, interpolate the values with the correlate of protection analysis, and then estimate an effectiveness because fundamental properties of the infection have changed between variants. Yet, here is how Novavax often presents their immunogenicity data to regulators:
And here is an example from a recent publication:
When presented in this manner, Novavax seems to attempt to imply that with their booster of ancestral vaccine, it has induced immune responses that approximate the same level of protection as those achieved in their initial trial against more recent variants, which is not a reasonable assumption (and this is absolutely the interpretation The Fandom has run away with and proselytized). I DO think it is reasonable to say that a booster dose of Nuvaxovid after a homologous primary series markedly boosts antibody responses against even BA.5, which is expected to greatly enhance protection, but presenting these metrics with a specific efficacy value (especially such a high one) is misleading.
In the same vein, Novavax published a short letter in the New England Journal of Medicine presenting antigenic cartography data showing the effect of successive doses of their vaccine on antigenic distance of SARS-CoV-2 variants:
The basic idea here is that the closer together the variants are on the antigenic map, the smaller the change in antibody titer is between them. Basically, as the antigenic distance between the variants gets closer to the ancestral variant (whose spike protein is used in the vaccine with the previously stated minor tweaks), the antibody response to these variants becomes more similar in magnitude to that of the ancestral variant, implying that the protection offered by the vaccine against these variants also increases. Novavax also gave the precise antibody titers here as well, which is great transparency on their part. But the way they discuss these findings is worth mentioning:
These data indicate that boosting with the NVX-CoV2373 vaccine resulted in enhanced cross-reactive immunity to SARS-CoV-2 variants, a decreased gap between immune recognition of the variants and the ancestral strain, and the induction of a potentially more universal-like response against SARS-CoV-2 variants. We believe that this phenomenon may be driven by the conserved epitopes found on the recombinant protein vaccine, whereby expression of the full-length trimers of the S protein present epitopes that are conserved across variants for recognition by the immune system.4
Did you see it? They just described immune imprinting. They are saying that their vaccine works so well because imprinting allows it to focus immune responses on well-conserved parts of the spike protein- the very thing mRNA vaccines were criticized for doing. Now, admittedly, Novavax is not responsible for the heat mRNA vaccines took on the phenomenon of imprinting, despite emerging evidence strongly indicating that this property of the immune system is far more positive than it is negative, so I don’t hold them at fault for that. However, some have taken this one figure and run away with the interpretation that Novavax is markedly superior in breadth to mRNA, which is not well supported. Furthermore, the timing at which samples are taken has an effect on antigenic cartography results- as you go further away from peak antibody titers, the antigenic distances have a tendency to increase, and no longitudinal antigenic cartography data are presented here. These limitations are not acknowledged.
In a recent letter, Novavax describes the IgG subclass composition of Novavax vaccinees. This concerns the finding that with successive doses of mRNA vaccines, there is an increasing proportion of the immune response to the spike protein that is comprised of IgG4. I have written extensively about the knowns and unknowns of this finding here. In this work, they write:
The clinical importance of SARS-CoV-2–specific Fc-mediated responses (i.e., ADCP, ADCD, and ADCC), which are poorly engaged by IgG4, is rapidly gaining appreciation.4 , 6, 7, 8, 10 Fcγ-dependent effector functions can provide additional mechanisms for virus control that may be complementary to neutralization, and these may be important for the promotion of vaccine-mediated cross-protection to evolving SARS-CoV-2 variants. 10 Here, we report that the NVX-CoV2373 rS protein vaccine does not appear to induce notable increases in IgG4, even after multiple exposures, or to impair Fcγ-dependent effector responses as observed with mRNA vaccines. Instead, NVX-CoV2373 drove proportional increases in IgG3, perhaps the most potent SARS-CoV-2 neutralizing antibody subclass,3 and enhanced surrogate ADCP, ADCD, and ADCC activity.
There is a lot to unpack here and it mainly has to do with omissions. To begin with, this data is relevant only for those who have had a Novavax COVID-19 vaccine for all of their doses. If you have received mRNA for your first doses, as a very large proportion of the general public has, everything written here has no relevance to you because class switching to IgG4 means that you cannot shift back to IgG1 or IgG3- in the process of making IgG4, you lose the DNA encoding these constant regions from the B cells. The only way to get IgG1/3 predominant responses at that point is to induce new, naive B cells against the spike protein. However, this is very difficult to do because the immune system has already solved the problem of how to deal with the spike protein by making IgG4 antibodies- you would need a spike protein that has a very high degree of escape from those IgG4 antibodies, which is very hard to do because these IgG4 antibodies have substantial binding breadth. That is NOT a bad thing- it means you have antibodies that cover tons of variants, even those that don’t yet exist. True- Novavax’s data definitely show that the antibodies elicited by their vaccine has greater Fc effector functions in their assay than do the mRNA vaccines and they specifically highlight the importance of IgG3 as a contributor to neutralization and these effector functions. An uninitiated reader might be forgiven for thinking that more effector functions would be better, but in fact this isn’t the case. As I explain in my post about IgG4, the presence of poorly neutralizing IgG antibodies that strongly drive activation through CD16A (FcγRIIIA) was able to predict 80% of the hospitalization risk from COVID-19, and IgG3 has the strongest binding to CD16A out of all subclasses. IgG3 is unique because it has a very large hinge region and is a very potent activator of complement. Complement is also implicated as being central in the immunopathology caused by severe COVID-19. It is true that owing to the flexibility of its hinge, IgG3 is better able to engage in bivalent binding which gives it greater potency of neutralization (this effect is even stronger for IgM because it ends up having 10 binding sites as it is typically present as a pentamer). However, IgG3 (and IgM) have much shorter half lives than the other subclasses of IgG (unless you are in the rare part of the population with allotypes of IgG3 where there is an R435H mutation) because they cannot efficiently bind FcRn at acidic pH (as encountered in recycling endosomes). IgG3 and IgM tend to be produced early on in the immune response because its potent effector functions are thought to buy time by helping to make an environment hostile to infection (at some expense of the host’s health) while affinity matured, class-switched IgG1/2/4 and IgA1/2 are being produced to control the infection. Making any of these other subclasses requires loss of the G3 constant region DNA that defines the properties of the IgG3 antibody. It is speculated that the reason most of the population lacks this allotype of IgG3 is because the very potent effector functions coupled with a long half-life could readily result in inflammation whose harmful effects on the host would be far more difficult to control. Beyond this, Novavax blithely chooses to ignore that the mRNA vaccines, regardless of their induction of IgG4, consistently show enhancement of protection with each booster and robust prevention against severe disease that appears to be durable.
If we however set aside all of the above for the moment, they do something here that is arguably far worse. Citation 8 in this letter goes to a review on IgG4 (which is essentially entirely anti-vaccine propaganda) in the predatory publisher MDPI written by, among other people, William (aka Villiam) Makis. Makis is a former Canadian nuclear medicine radiologist from Canada whose medical license is now inactive following disciplinary action in 2017 as a result of unprofessional conduct and was declared a vexatious litigant. You might however mistakenly think that he is an oncologist (and to be clear: not only isn’t he an oncologist by virtue of the fact that he has no license to practice medicine but he has never practiced as one and does not have the qualifications for it) because he incessantly pretends to be one on social media, and furthermore is a massive proponent of the turbo cancer and died suddenly hoaxes. Okay, but what’s the big deal here? Is Novavax supposed to know every single anti-vaccine propagandist on the planet and make a concerted effort to avoid using their work? I would argue yes. There honestly aren’t that many of them and as makers of a vaccine, I do not find it plausible that they would not know who is responsible for hurting sales and uptake of vaccines, but even if I am to give them a pass on this point, the paper is filled with red flags, and it is not believable to me that Novavax’s staff would not catch these. None of these authors is affiliated with an immunology, allergy, rheumatology, or infectious diseases department even though IgG4 is a very niche area within immunology. The paper immediately conflates total IgG4 for spike-specific IgG4 in the abstract. The introduction immediately alleges that third doses of vaccine cause more harm than second doses on the basis of a cherry picked ONS report. Since the seminal paper was published in December 2022 in Science Immunology, Pubmed has indexed hundreds of reviews on IgG4, including one in a premier review journal, Nature Reviews Immunology, written by one of the most published scholars in the field of IgG4. Yet, rather than cite any of these, they made the conscious choice to use the ethos they hold as a legitimate body of scientific expertise to elevate work whose transparent intent is to foment a narrative that vaccination is harmful, one where an author directly profits off of supplement sales intended to combat purported toxicity of vaccination. Beyond that, is this citation even necessary given all the other citations made in the exact same place that make the exact same points? Who we cite and how we cite them matters, and Novavax’s decision to elevate this work as legitimate out of all of the literature available to try to make their vaccine look better is absolutely reprehensible.
I don’t plan on taking their vaccine because I’ve yet to see any advantages for myself given the data we have and the fact that none of my mRNA vaccine doses have been tough to handle, but I am especially resistant to contribute to the remuneration of an organization that is either this careless or this indifferent to the consequences of its behavior.
The 2024-2025 Season COVID-19 Vaccine
Novavax presented data on a JN.1 booster in animals at the VRBPAC meeting on June 5, 2024. Novavax is more constrained by the technology they use- protein vaccines take longer to update than mRNA. Thus though JN.1 itself has now been replaced with descendant variants dubbed in some sources as FLiRT variants, Novavax is not able to make a vaccine targeting one of these variants in time for distribution in the 2024-2025 season, but was able to do so for a JN.1 vaccine. Basically, Novavax showed that current variants are antigenically similar to one another and close to JN.1, which supports the use of a JN.1-targeted vaccine (true). In particular, some of the most important data came from nonhuman primates that had received 2 doses of their XBB.1.5-flavored vaccine and then were boosted with their JN.1 vaccine after 11 months. The specific values of titers here are hard to interpret because there is no benchmark for them (e.g., a cohort of nonhuman primates that received XBB.1.5 mRNA vaccines and then JN.1 mRNA). In brief, a JN.1 booster to these primates does markedly increase pseudovirus neutralizing antibody titer against current variants, including FLiRT and FLuQE variants, with titers all falling in the same ballpark.
This was also done with mice receiving a JN.1 booster 2 months after a 2-dose series of XBB.1.5 vaccine with similar results:
CD4 T cell responses were also measured and as expected, they are similar across variants:
In brief, the immunogenicity data Novavax presented support that a JN.1 vaccine is a good update for this current season.
There are some people attempting to litigate whether or not this is a better choice than KP.2. Proponents of JN.1 spike contend that JN.1 represents a genetic root for current variants, and so it is a better choice because the path of antigenic drift that SARS-CoV-2 will follow is not readily predictable. Some have also argued that Moderna’s JN.1 vaccine performed better in animals than their KP.2 vaccine against KP.3. This point is true but it is noteworthy that the exact opposite is true of Pfizer, with KP.2 showing in particular better titers against FLuQE variants. The discordance in these results may be because the animals had different immune histories- Moderna’s were primed with 2 doses of XBB.1.5 vaccine and then boosted with JN.1 or KP.2. Pfizer’s received 2 doses of ancestral, BA.4/5 bivalent, and then either XBB.1.5, JN.1, or KP.2. As our own immune histories have gotten more complex, modeling them with animals has gotten to be more challenging, but in most cases, people’s first exposures were variants that had ancestral spike or spike proteins very similar to it, and we understand that the first spike we are exposed to influences the choice of antibodies used against future spikes which share structural features with the first one (aka imprinting). Because of this, I am inclined to believe that Pfizer’s vaccination regimen is the more relevant one for most of the public as far as expectations of the immune response of KP.2 vs. JN.1 spikes. Furthermore, my personal perspective is that while Novavax is correct that the difference in antigenic difference is minimal between JN.1 and KP.2, I prefer to go farther from the antigenic root as this has been shown consistently to result in broader antibody responses (yet again, I sit disappointed that we don’t have a vaccine that incorporates spike from SARS-CoV-1). Nonetheless, Novavax’s vaccine does show comparably good neutralization for a JN.1 spike across relevant variants, so I don’t think this consideration (KP.2 vs JN.1) is especially salient.
Data Conclusions and Knowledge Gaps
Novavax’s Nuvaxovid is a good vaccine against COVID-19 and a valid option for vaccination, particularly for those who struggle with mRNA vaccines’ reactogenicity. There isn’t any strong evidence that Nuvaxovid offers better protection than mRNA vaccines against COVID-19. The immune responses seem to be on par with mRNA when it comes to the antibody and CD4 T cell response, but the vaccine clearly loses on CD8 T cell responses. The exact significance of that is not totally clear. Novavax seems to continue to be held back by its failures to appropriately scale up production, but these maybe might get resolved soon. In terms of questions that are still either open or incompletely answered, I would say we lack clarity on the following:
How does the durability of Novavax compare with that of other COVID-19 vaccines?
How does the quality of protection from Novavax compare with other COVID-19 vaccines?
How does the safety profile compare to that of other vaccines, specifically when considering rare adverse events?
I feel obligated to mention that none of my vaccine doses (all of which have been Pfizer) have given me worse than a sore arm, so I do not find this compelling as a reason to take Novavax as an individual. Nevertheless, my experience is not everyone’s and if the choice is Novavax or nothing, Novavax is the clear best choice.
This is even more money than Moderna received; the only OWS awardee to receive more money was Sanofi/GSK for the vaccine that ended up being Vidprevtyn Beta, a protein-based vaccine adjuvanted with AS03. This vaccine was approved in the EU and UK, but, ironically, not in the US. The manufacturer (Sanofi Pasteur) has discontinued the vaccine for commercial reasons.
One point that I feel is very important to emphasize: there are a lot of variations in the antibody assays used by different groups to measure immune responses. These are not directly comparable, by which I mean: you cannot say that a bigger number on one assay means a bigger response on another assay that showed a smaller number, especially if the metric being reported is not the same. Assays need to be standardized to make these comparisons, and ideally should be performed by the same individual in the same manner each time. The same exact sample can give very different titers depending on the type of assay used.
There is a perspective out there that argues that free S1 protein released into the circulation is a contributor to adverse events after vaccination; in brief, I find these claims unconvincing. They are predicated on the belief that there is extensive distribution of the lipid nanoparticles from the injection site, which is not substantiated. Preclinical models giving rats doses of vaccine in far excess of the equivalent amount from humans show that it is overwhelmingly retained in the injection site, though a portion of it does end up in the circulation which allows it to distribute widely. S1 shed in the circulation could conceivably end up widely distributed through the body, subject to the constraints of certain anatomical barriers such as the blood-brain barrier, but the appearance of S1 in circulation is transient, and the concentrations at play are about 100,000 times lower (Kd of spike-ACE2 interaction is 120 nM, dose of spike protein found in circulation after mRNA-1273 vaccination is 1.5 pM) than the concentrations that would be expected to induce any kind of meaningful signaling through e.g., ACE2.
In the same vein, there is work that detected the presence of full-length spike protein unbound by antibodies in patients who developed vaccine-associated myocarditis; however, this does not give insight into the direction of causality (i.e., is there myocarditis because of elevated presence of this spike protein in the blood, or is the immune response causing the myocarditis resulting in increased presence of spike in the blood?).
I should mention however that glycans that come from other species are extremely immunogenic. For example, catarrhines (Old World monkeys, apes, and humans) lack a functional version of the enzyme glycoprotein alpha-galactosyltransferase 1 (GGTA1), which is responsible for making Galactose α-1,3-galactose (aka α-gal or Galili antigen). This means all of our proteins lack α-gal. However, this enzyme is functional and extensively present in most mammals. It turns out, if you are bitten by the lone star tick or castor bean tick, you can end up making IgE antibodies against α-gal (it remains unclear why IgE antibodies develop as first principles immunology dictates that α-gal should only be able to produce IgM antibodies, though this assumes that α-gal is present by itself and not attached to a protein). If this occurs, the result is that you may become allergic to all red meat and it is known as α-gal syndrome (AGS). Antibodies against α-gal can also cause rejection of organs from other species which has resulted in the genetic engineering of animals that lack α-gal (that can also be used as a food source for humans with AGS).
Surprisingly, antibodies against α-gal might actually have an important survival advantage: If a tick has a blood meal (although some evidence suggests it might not even need to to introduce α-gal into your body) and then proceeds to bite you, the tick could act as a vector to transmit any number of novel viruses you have never encountered and render you sick. However, because these viruses were made (presumably) in a non-catarrhine mammal, antibodies against α-gal can rapidly bind to them and clear them before they have a chance to cause disease.
This also suggests that antibodies against α-gal can be induced as part of a generalized strategy against tick-borne viruses (so long as they are not of the IgE isotype). Furthermore, some other microbes also use α-gal as part of their proteins, meaning these antibodies can hold additional value beyond this.
It is also worth mentioning that glycan shielding is not uniformly bad when it comes to eliciting vaccine immune responses. Because the glycans themselves are not immunogenic (assuming they come from human cells or cells with human glycosylation machinery), they can be used to focus immune responses on specific parts of a viral protein by concealing the other parts. This is a potential option for dealing with things like deleterious immune imprinting (deleting the conserved parts is also an option, but this approach would allow you to keep your T cell epitopes to promote the immune response through them).
The exact reason that a reduced glycan shield results in a broader antibody response is not well understood. It is true that by reducing the glycosylation of the protein, more of it is being exposed, which means antibodies can now access it more easily. However, it is not obvious why this would result in better antibody responses because, when the protein occurs in the actual virus, while there can be variation in the efficiency of glycosylation at any given site, the glycan shield generally is present.
In one study using mRNA vaccines that encoded spike with glycosite deletions, it was found that this resulted in induction of the unfolded protein response, which may have contributed an adjuvant effect that promoted the induction of robust antibodies. The problem is that this cannot explain why a broader response when glycan-trimmed proteins are supplied directly, and yet this is also observed.
It is possible that the protein regions below the glycan shield may be accessible during brief periods of time as the protein naturally moves, and this can be exploited when high-affinity antibodies against those regions are already present. However, if the antibodies are not present already, exposure is too brief for binding to occur and thus these epitopes are not targeted. If this is true, it is also likely that these epitopes are not under strong selection pressure to mutate (but that might change if antibodies that bound strongly to them appeared without adequate suppression of transmission).
Another possibility has to do with a directly immunomodulatory effect of the glycans. For example, siglecs are a family of proteins that recognize glycans containing sialic acid, and exert an immunosuppressive effect. If viral proteins end up sialylated, this could hamper induction of immune responses against them. SARS-CoV-2 spike was in fact shown to evolve a binding site for Siglec-9, and mutating it away greatly enhanced antibody responses.
There has been some confusion about this so I want to clarify that other vaccines, such as the mRNA vaccines, also have adjuvants. In the case of mRNA vaccines, adjuvanticity arises from the lipid nanoparticle’s ionizable lipid and from the mRNA itself. Because the mRNA itself has adjuvant properties, these vaccines are sometimes described as being self-adjuvanting.
The natural biosynthetic pathway for QS-21 by the soap bark tree (Quillaja saponaria) has been published:
In the process, the team uncovered the biosynthetic pathway for another compound within the soap bark tree, QS-7.
AS01 is available at two dosage forms:
AS01B: gE/AS01B; GlaxoSmithKline Vaccines; 1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS-21
AS01E: gE/AS01E; GlaxoSmithKline Vaccines; 500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS-21
The soap bark tree as a source of QS-21 is limited in part because extraction from the tree destroys the bark, and trees must be 30-50 years old to produce QS-21. Under the method described, yield is lower from the YL-46 than from the soap bark tree, but production speed is faster by a factor of about 1000. With continued advancements in strain selection, directed evolution of enzymes, etc. this can likely be significantly improved even further.
This is commonly the case for vaccine adjuvants, and even true of aluminum salts which have nearly a century of use. Mechanisms are valuable to understand how to make better, more effective vaccines, but safety records speak for themselves.
I would hypothesize based on the proposed mechanism by which QS-21 is able to induce cross-presentation that more effective cross-presentation could be achieved by physical linkage of the antigen with the QS-21 adjuvant, so that they necessarily are present within the same endosome.
The major work describing possibility demonstrates an outdated understanding of immunology, referring to Th1 as pro-inflammatory while Th2 is anti-inflammatory; however, the experiments defining structure-activity relationships described herein seem methodologically valid.
This is intriguing because nanovaccines which incorporate agents to suppress the action of subcapsular sinus macrophages have shown 30-fold enhancement in neutralizing antibody titer and 60-fold enhancement of antigen-specific antibody when compared with nanovaccines alone, suggesting that barriers to lymphatic transport of antigen into follicles represent a substantial impediment to the generation of robust immunogenicity.
To be clear, I don’t mean people who generally just like Nuvaxovid or prefer it over other vaccine types because it has a more favorable reactogenicity profile relative to mRNA. I mean specifically people who think and behave as though Novavax’s vaccine is somehow magic and vastly superior in its protective effects than any other vaccine available for COVID-19.


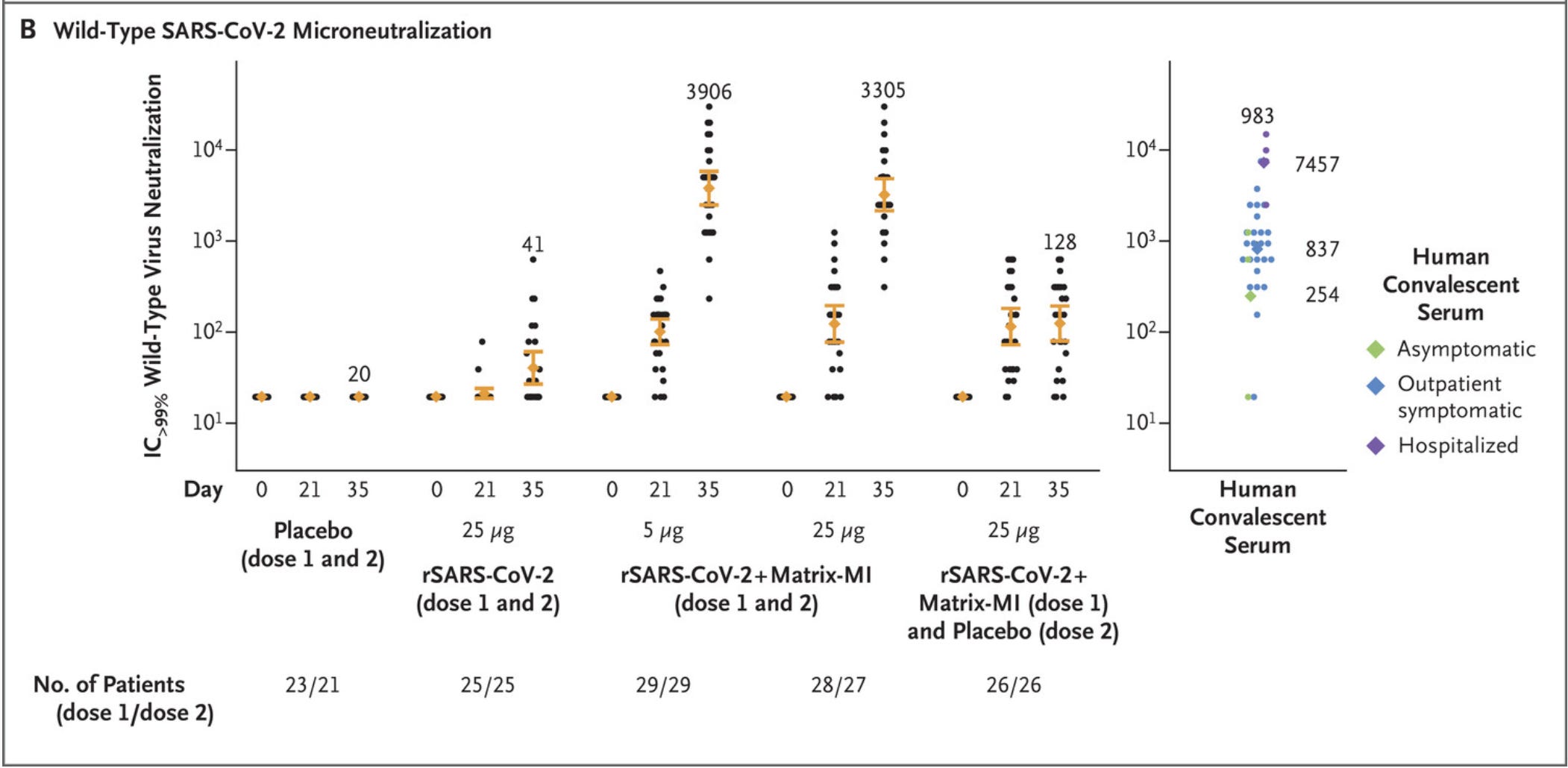

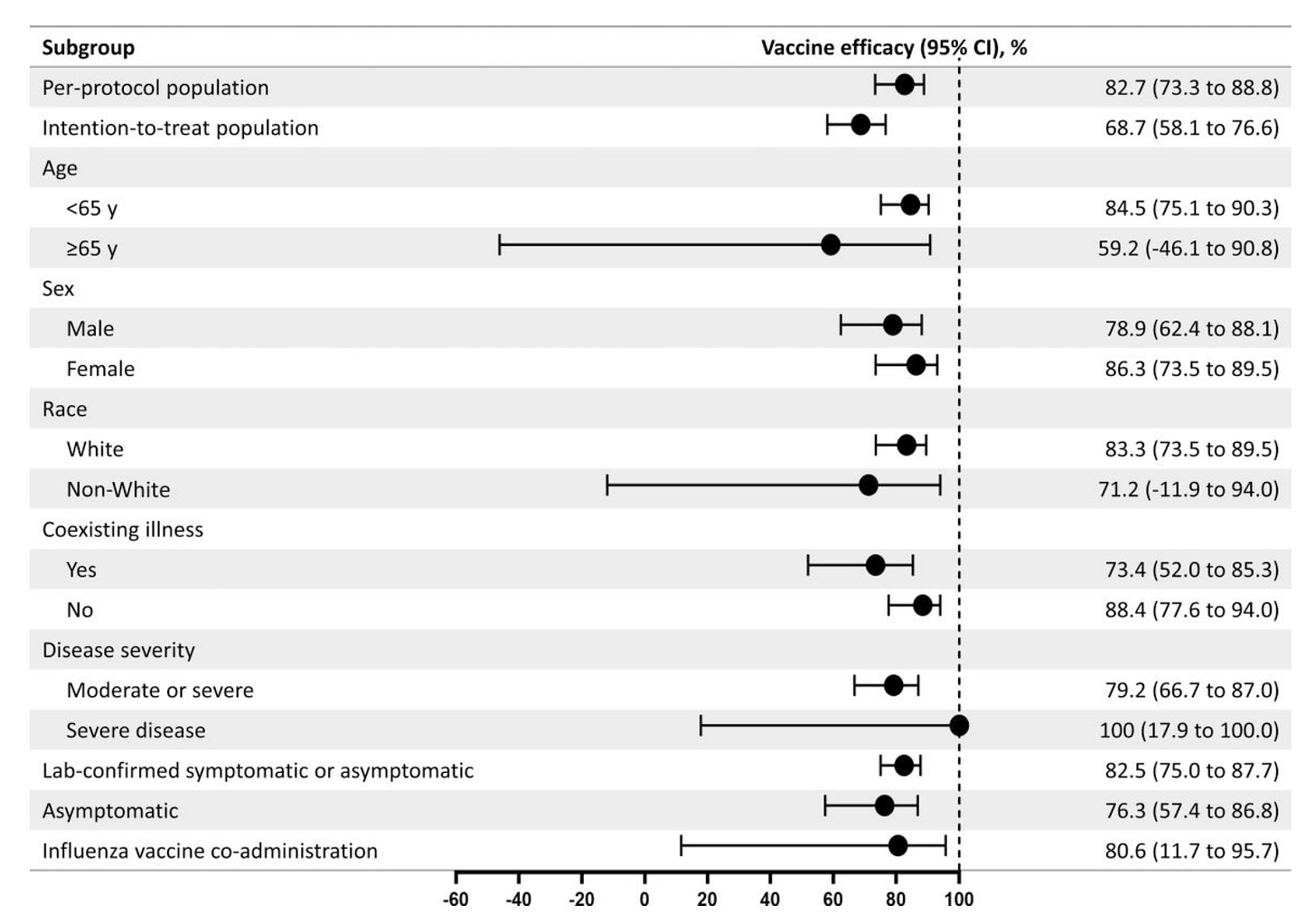
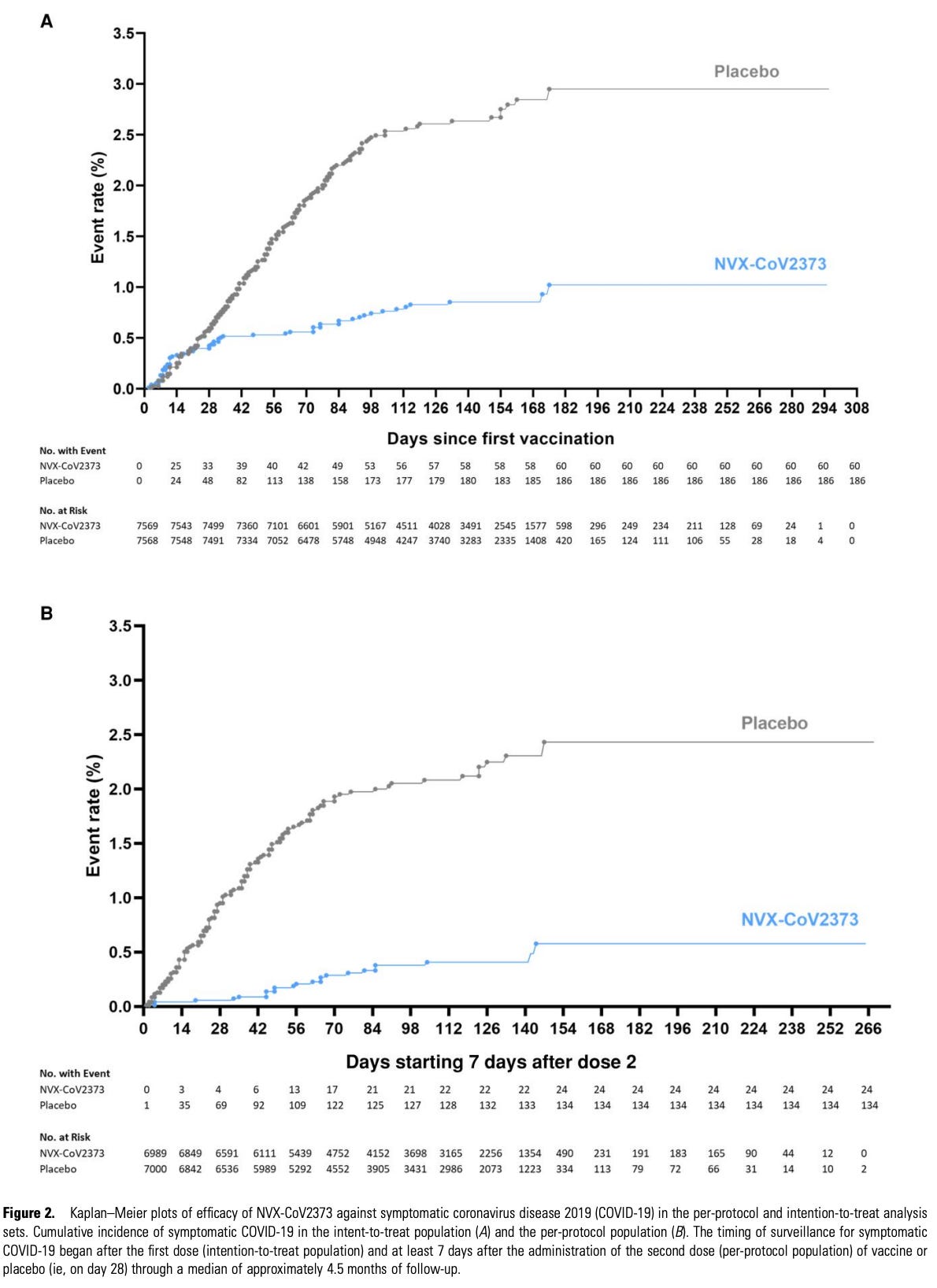

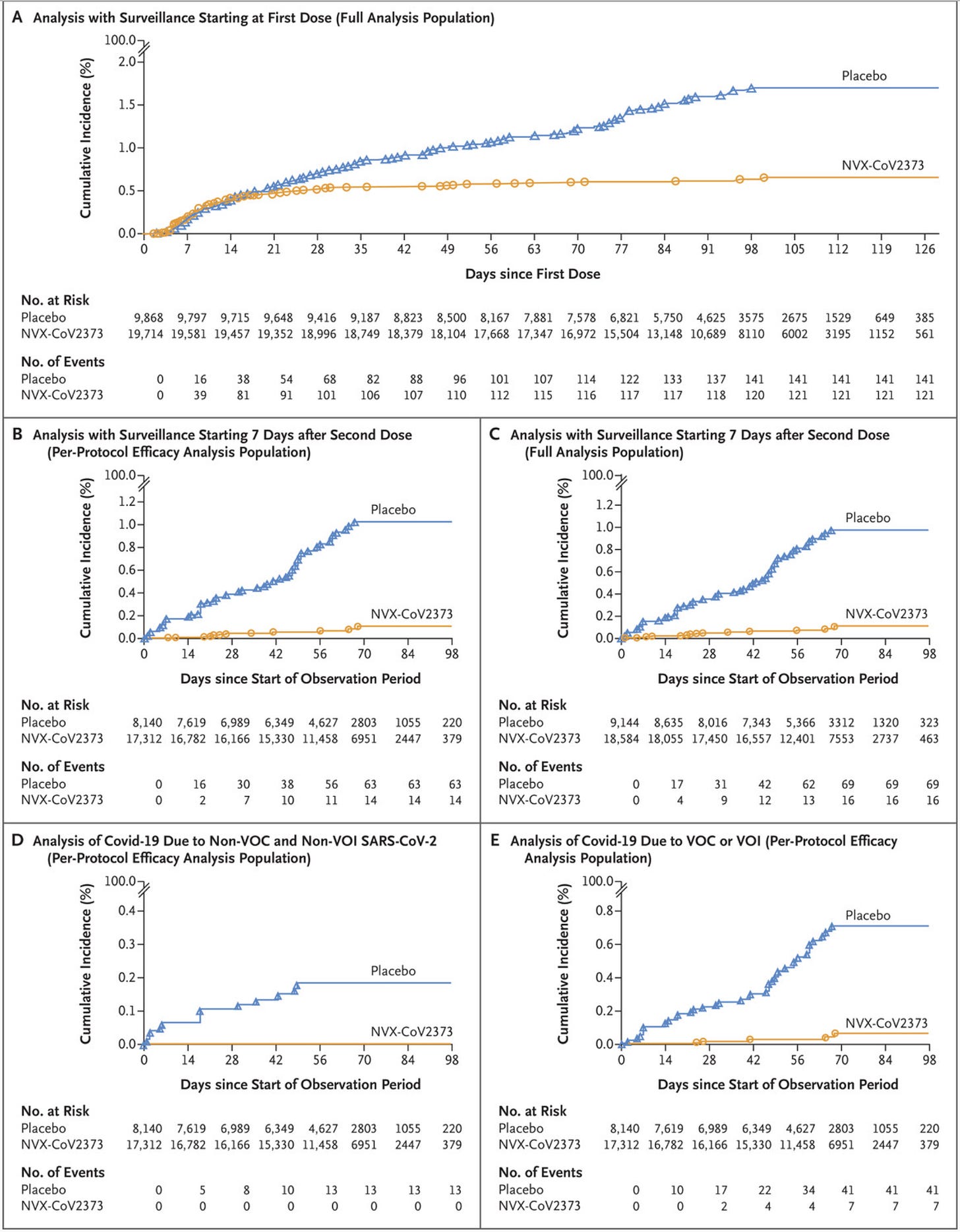
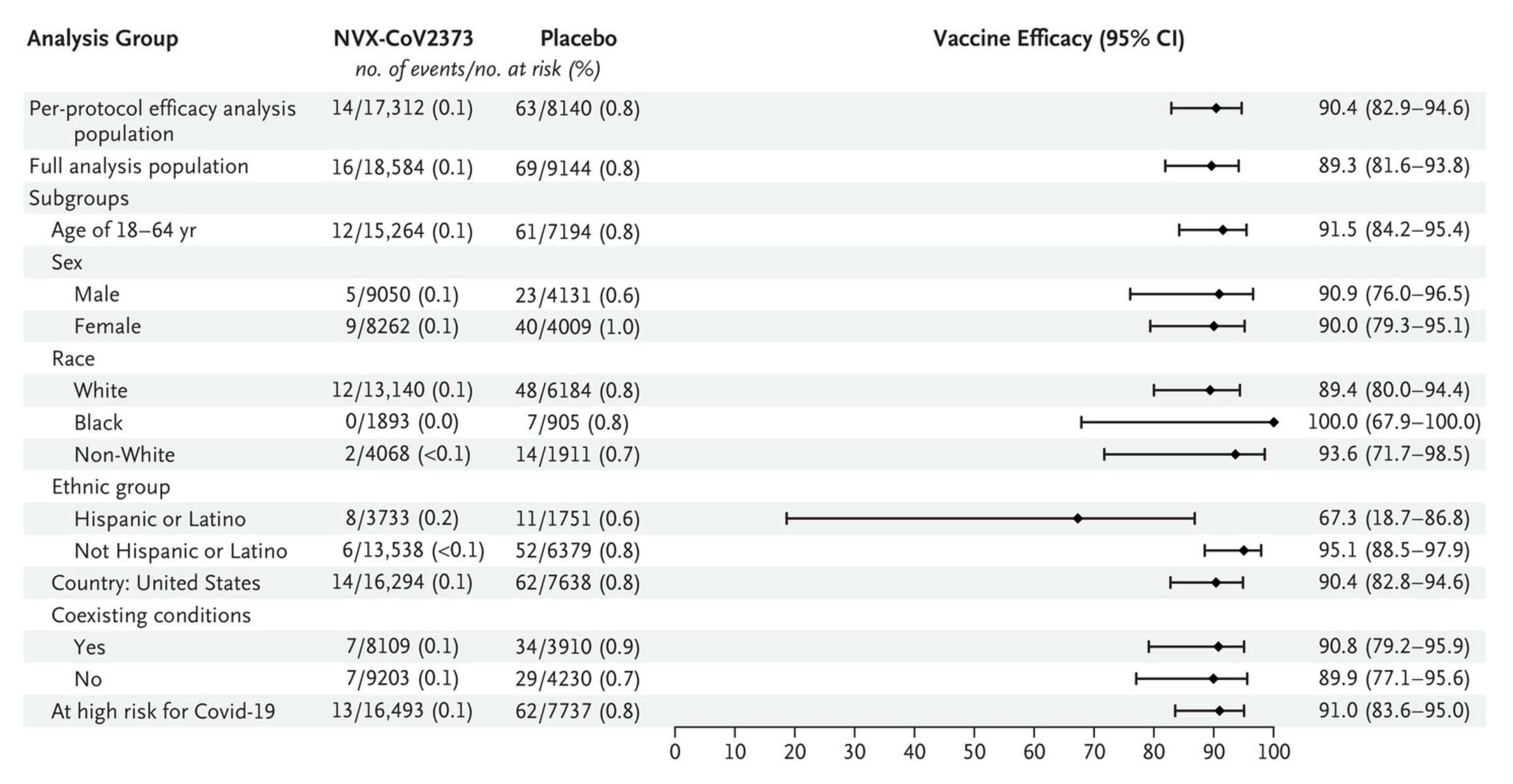
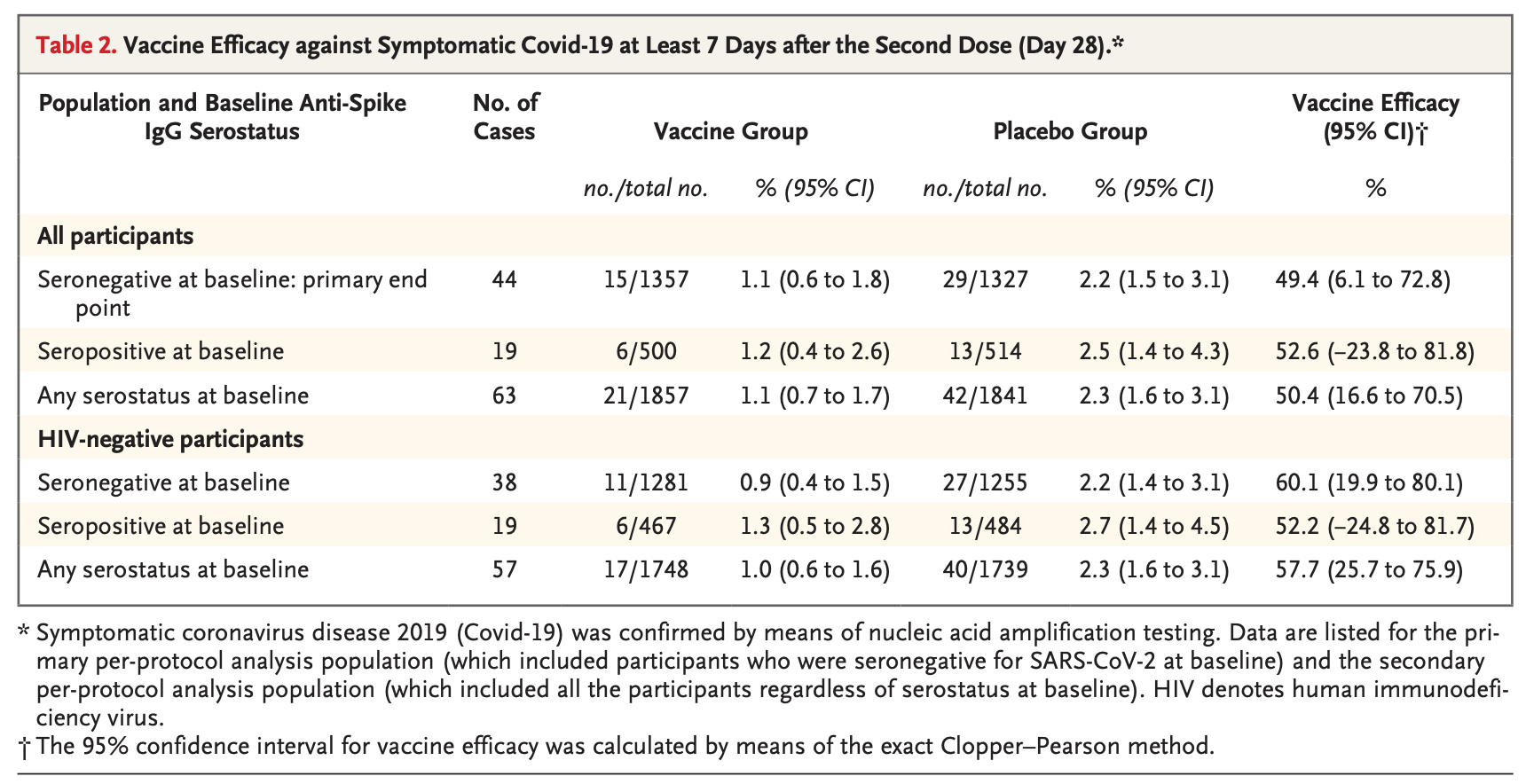
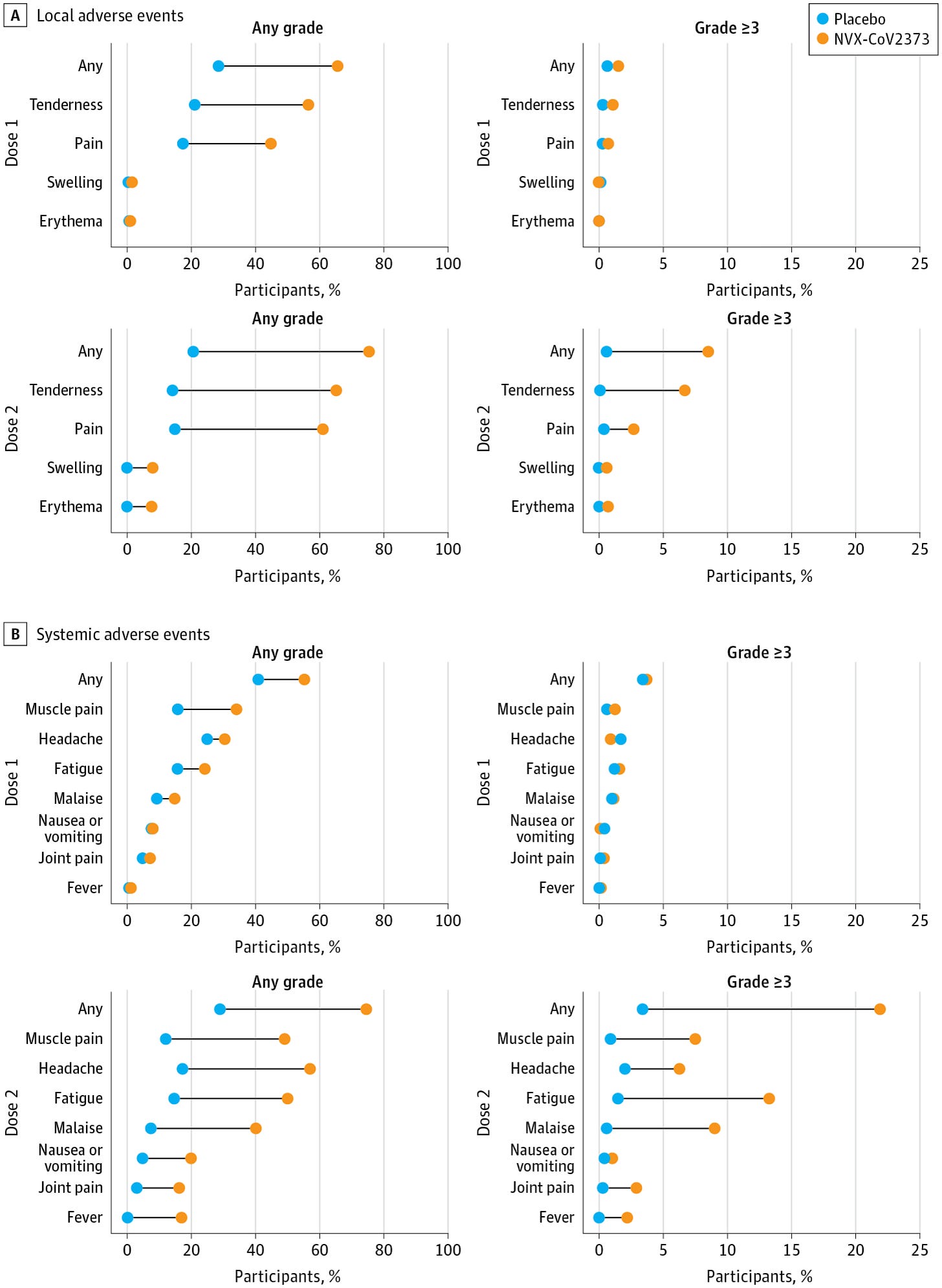
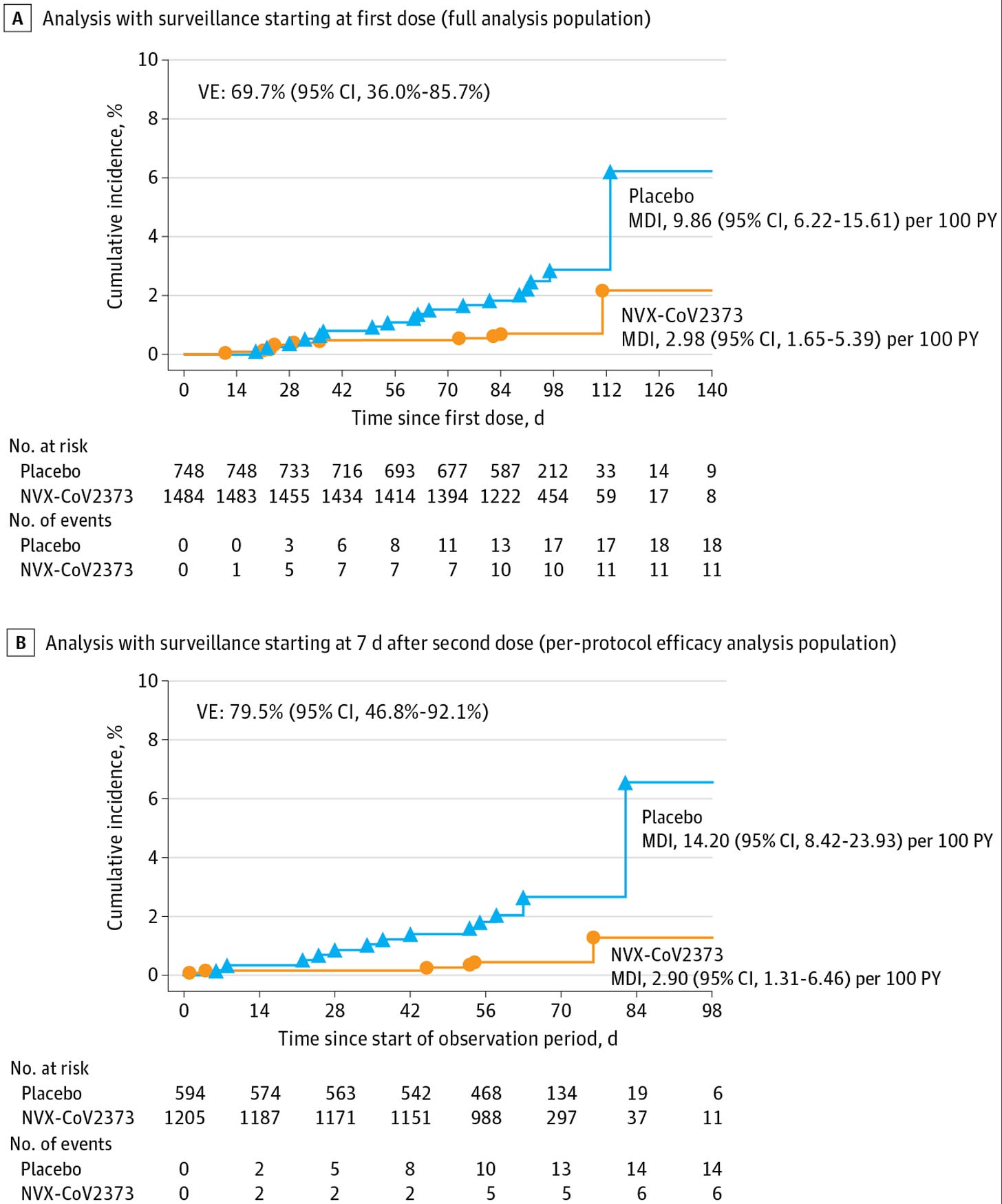


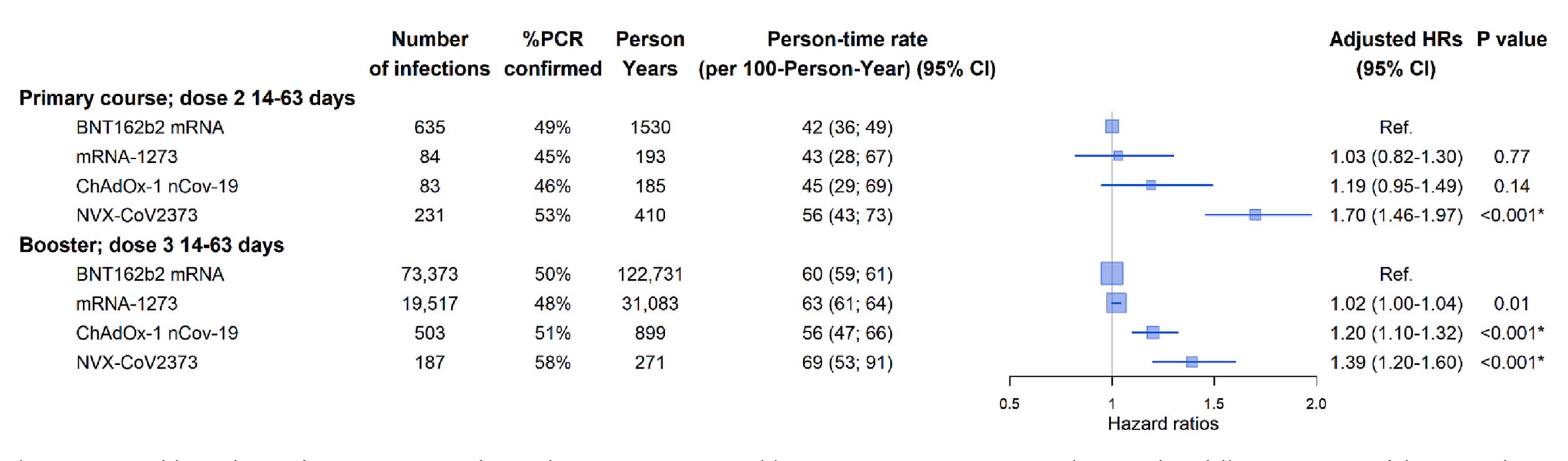
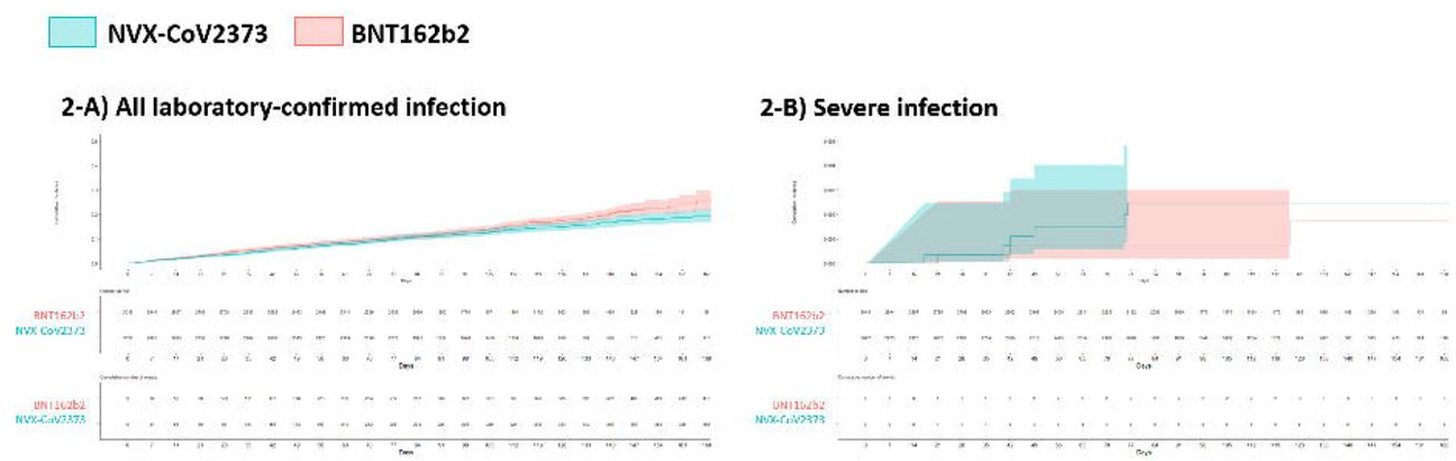
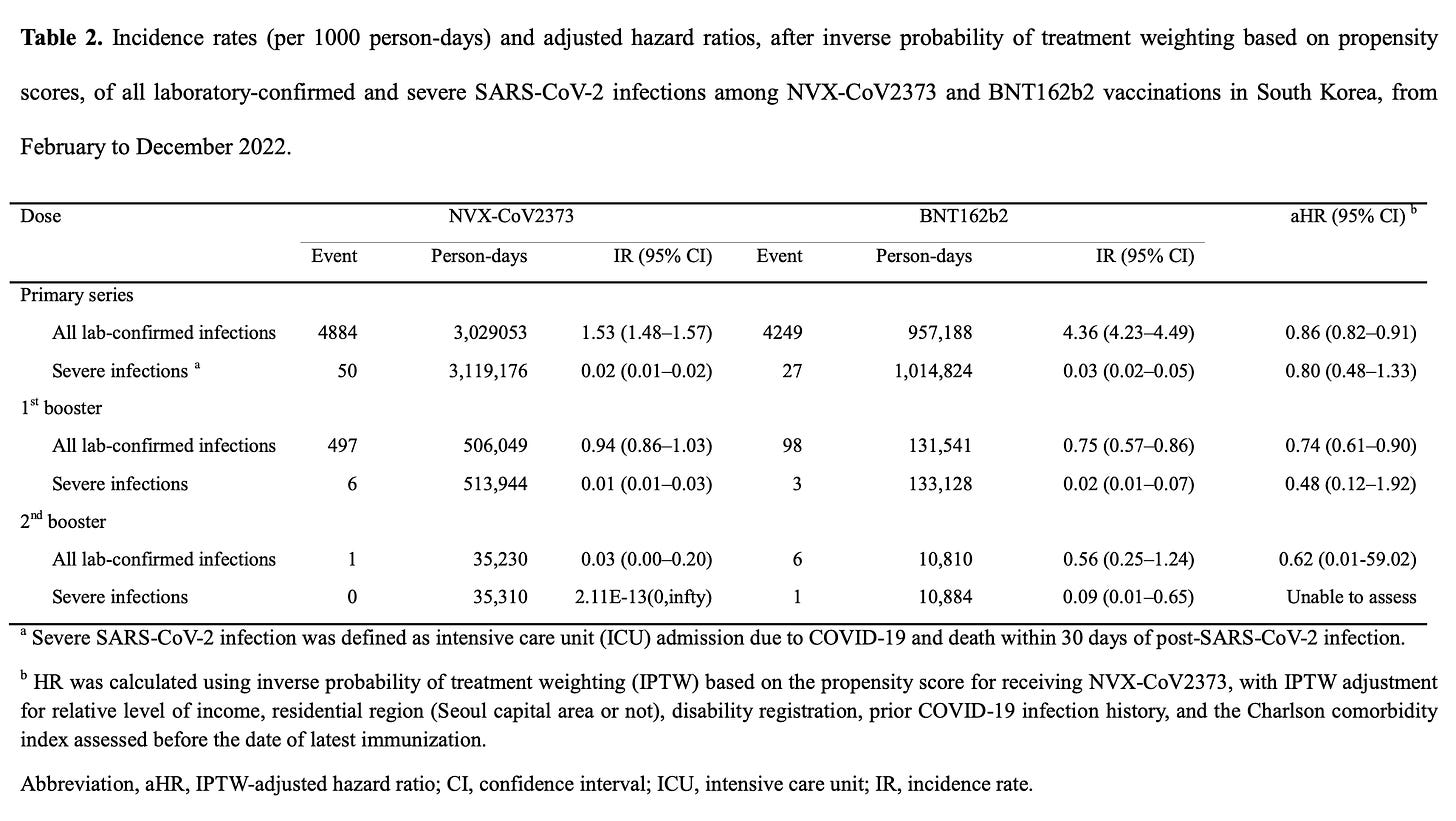
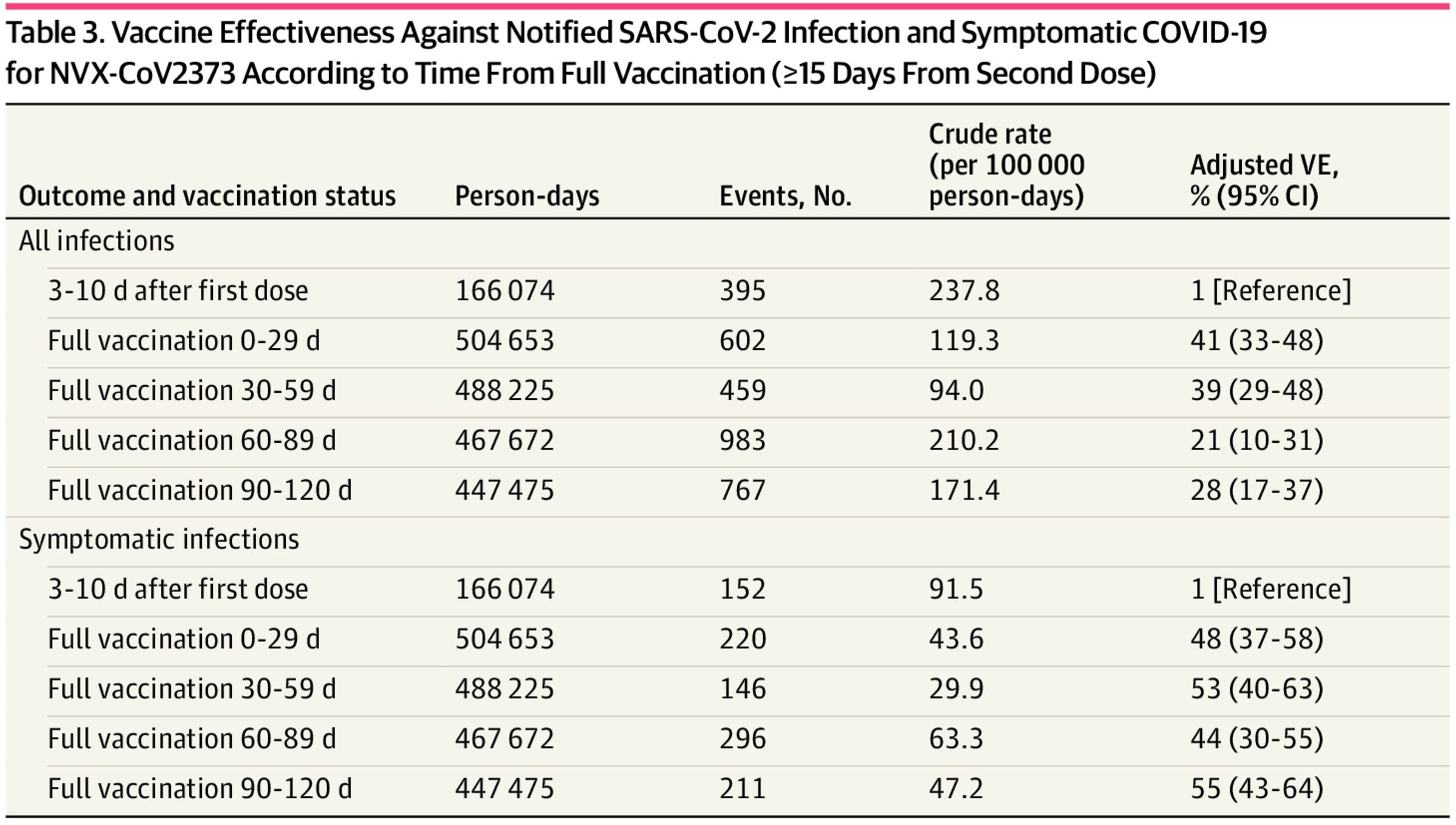
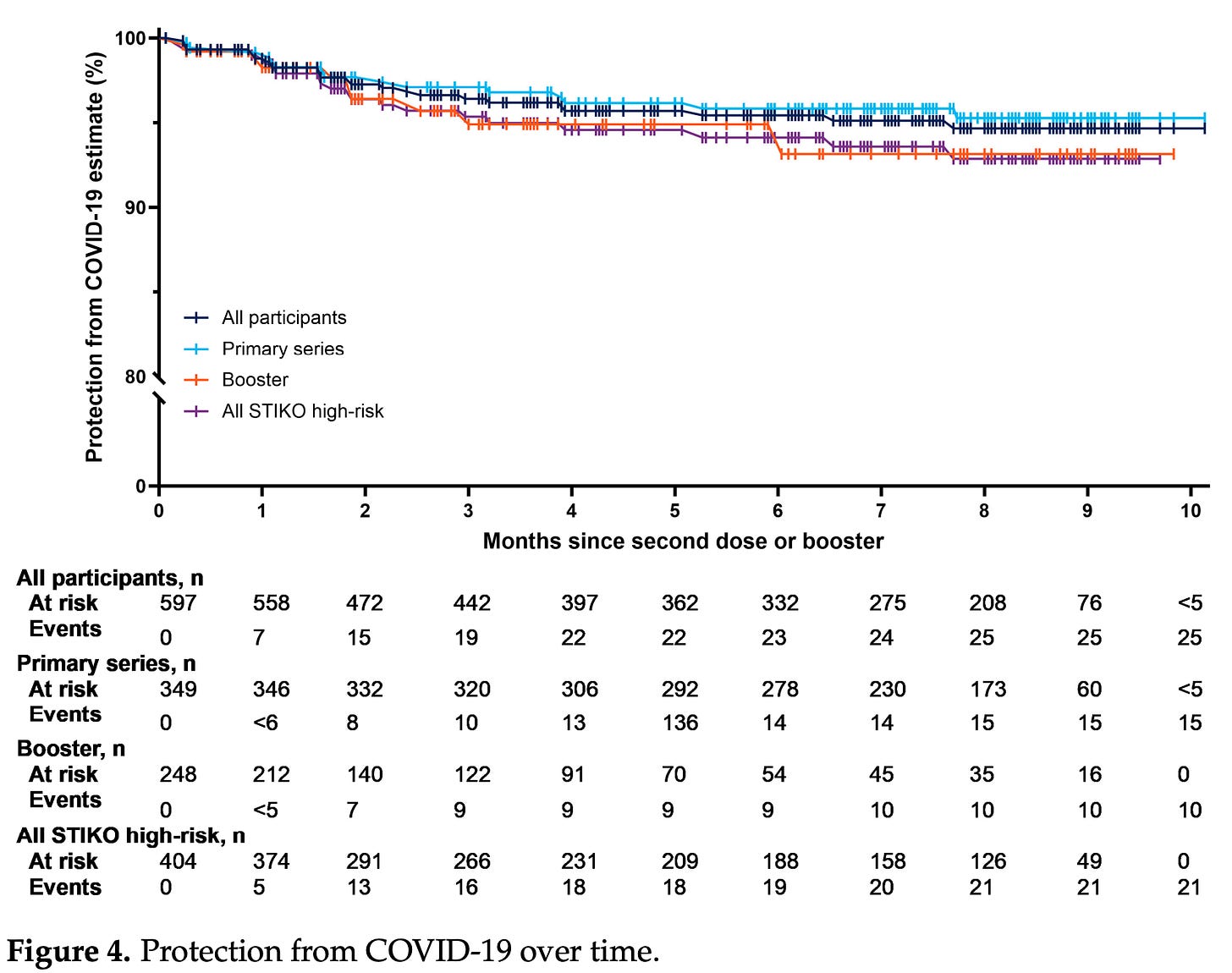

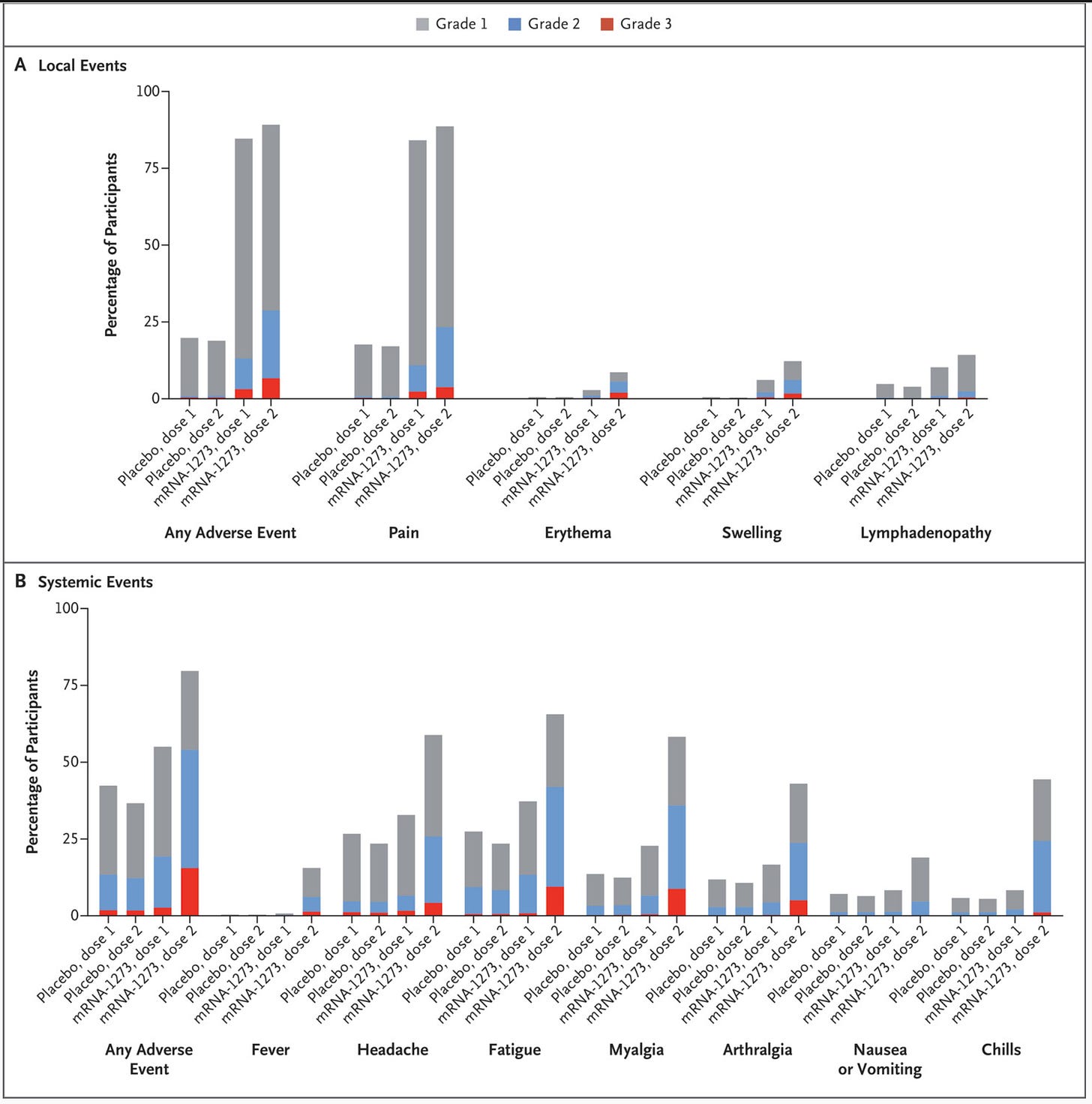
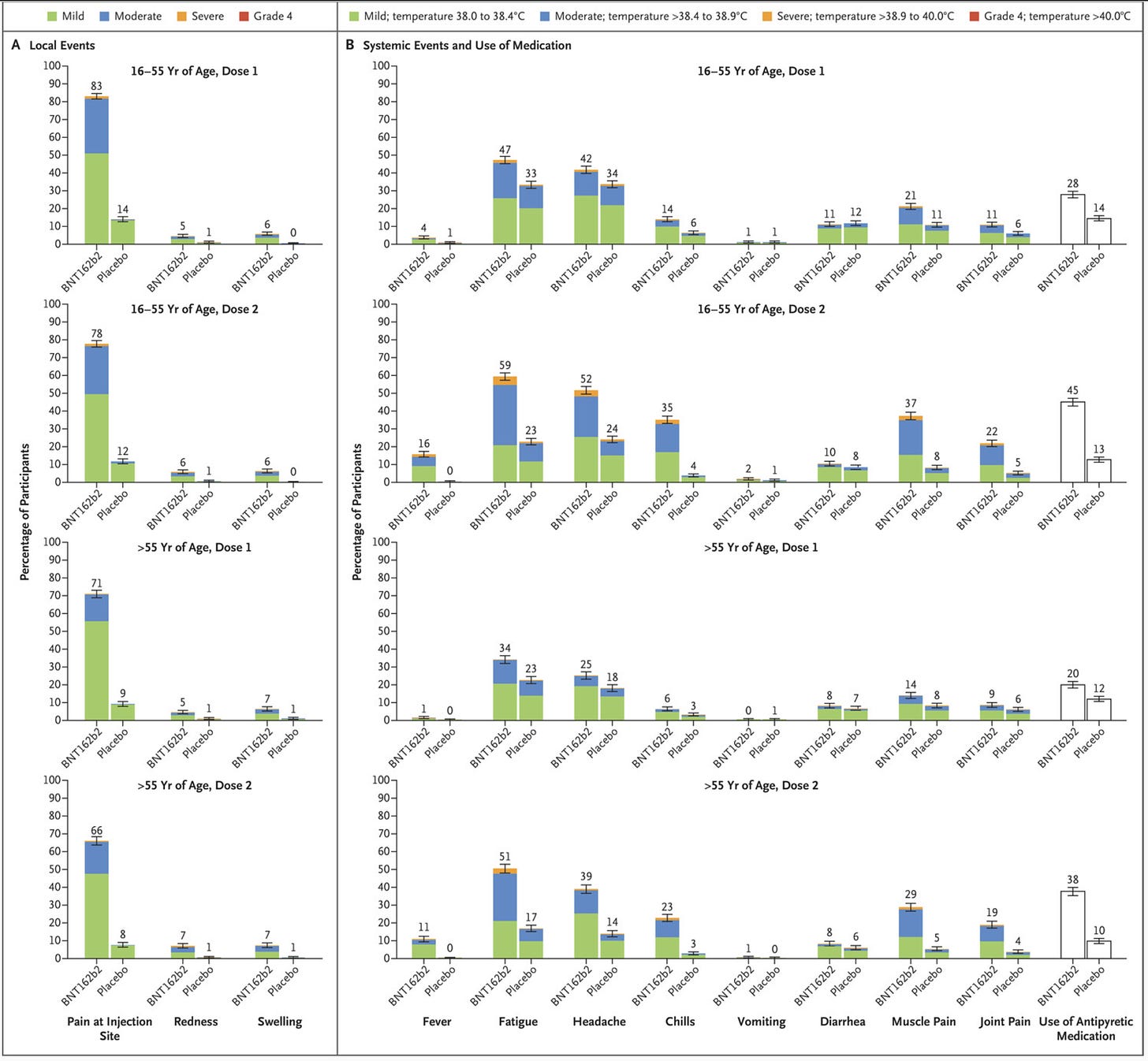


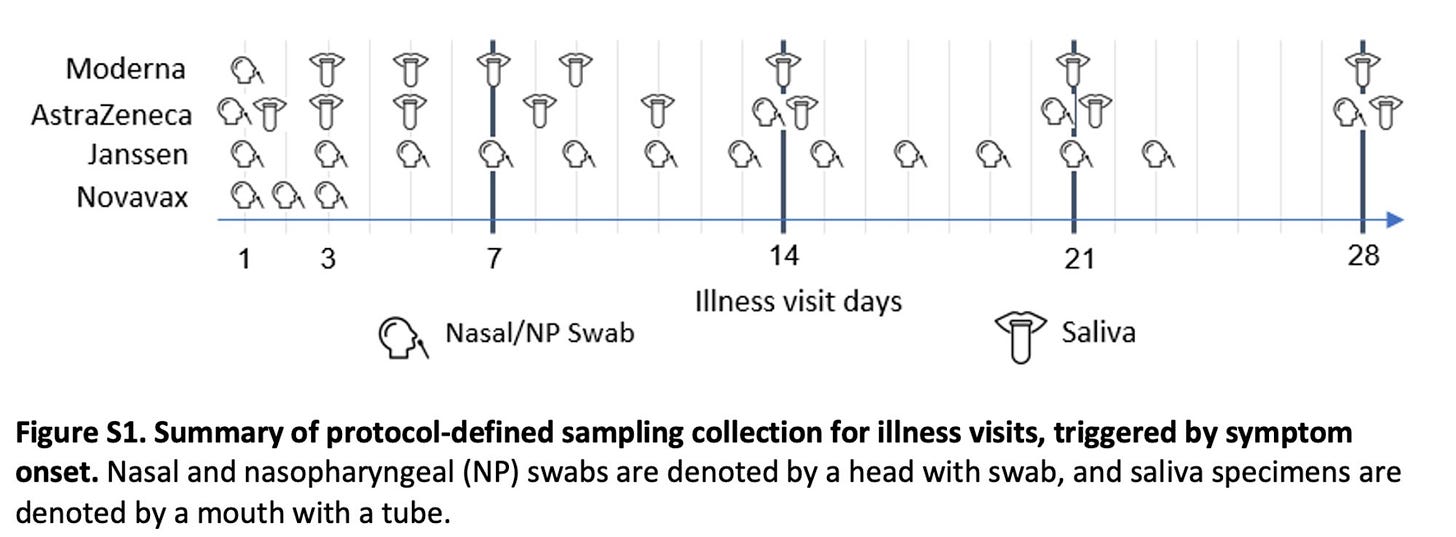
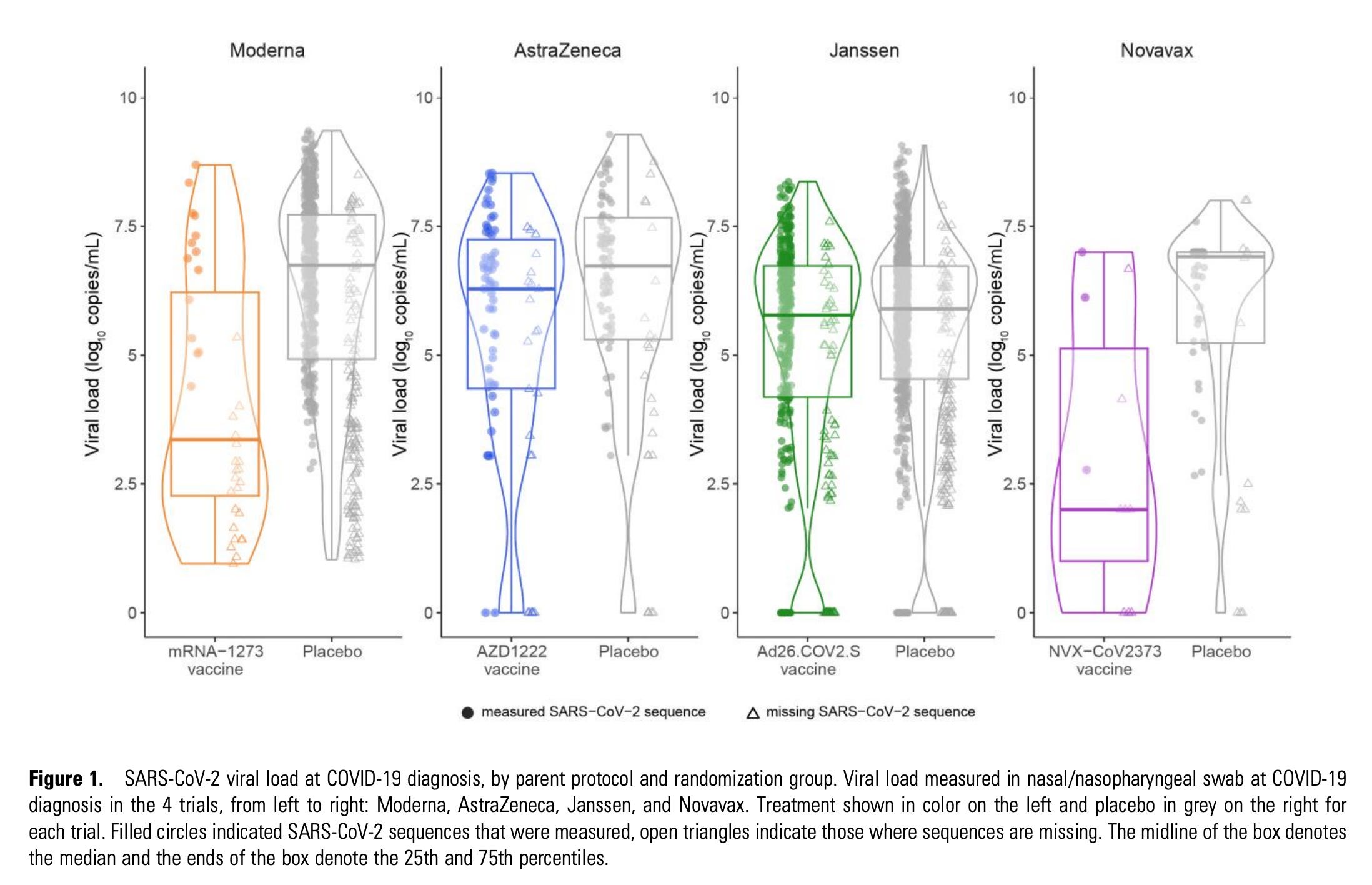
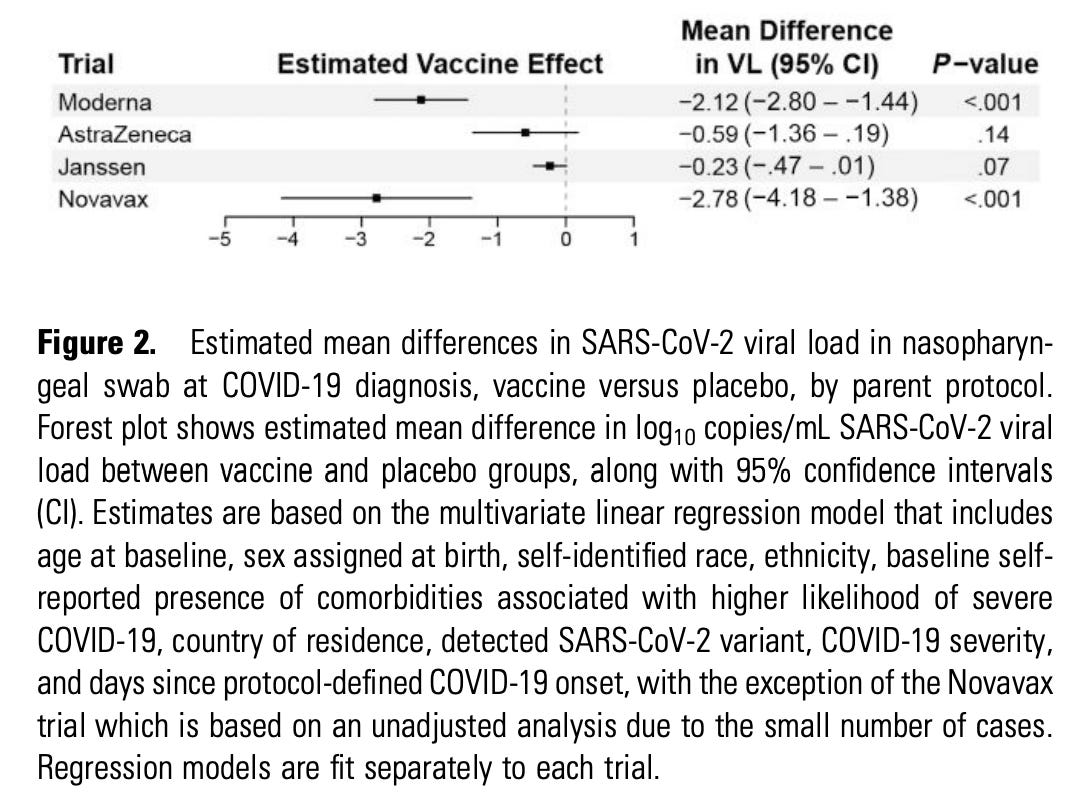



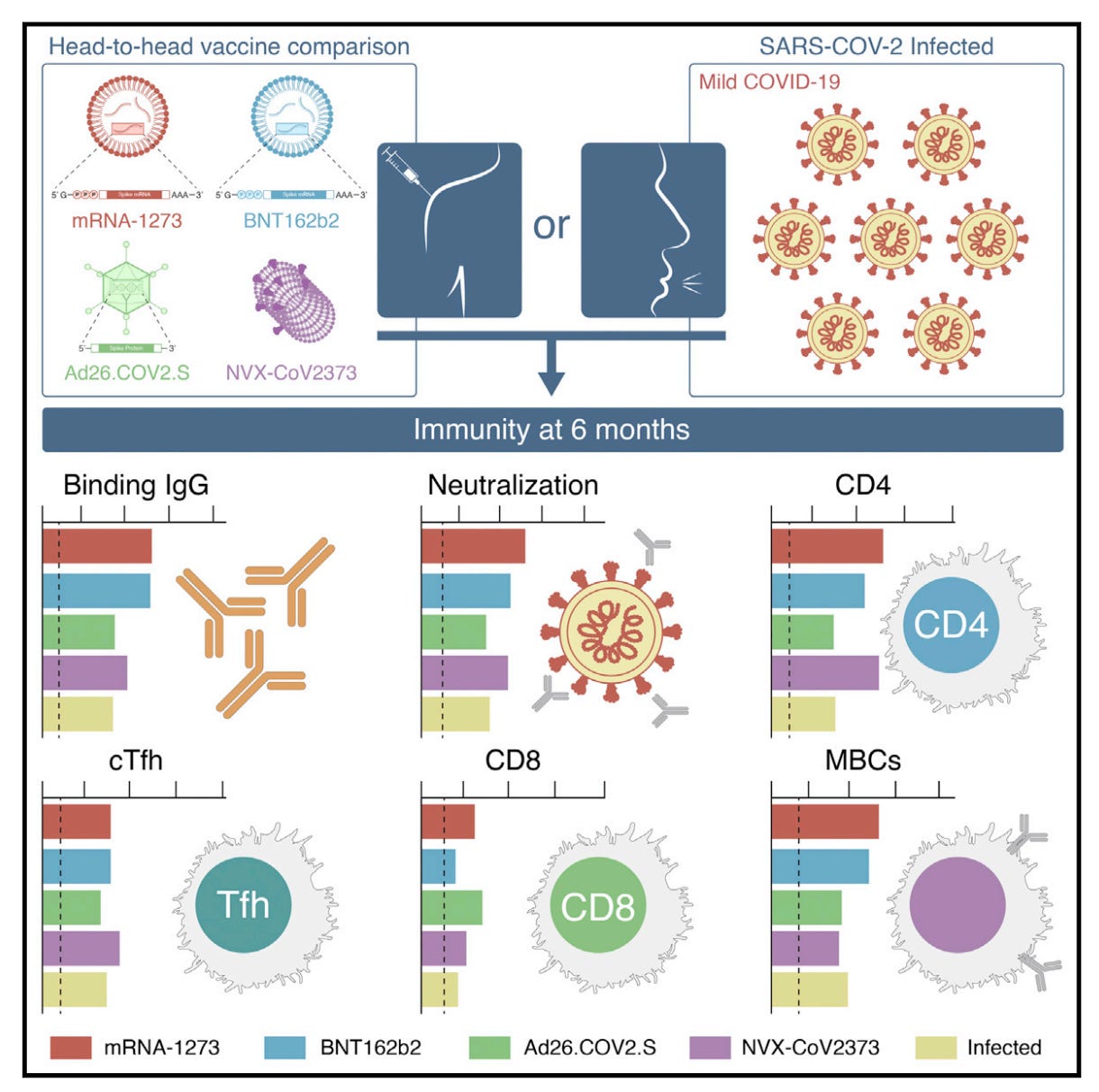

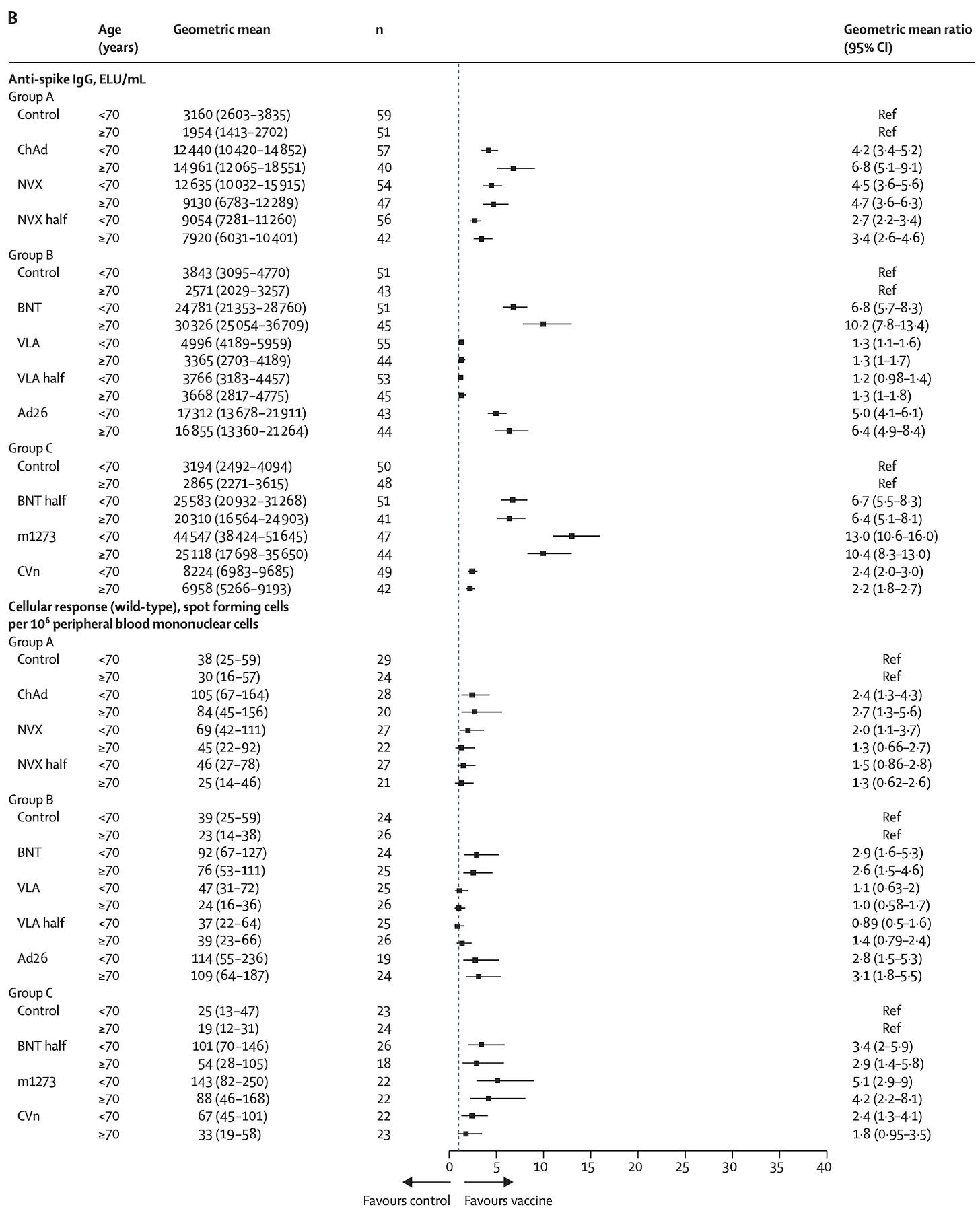
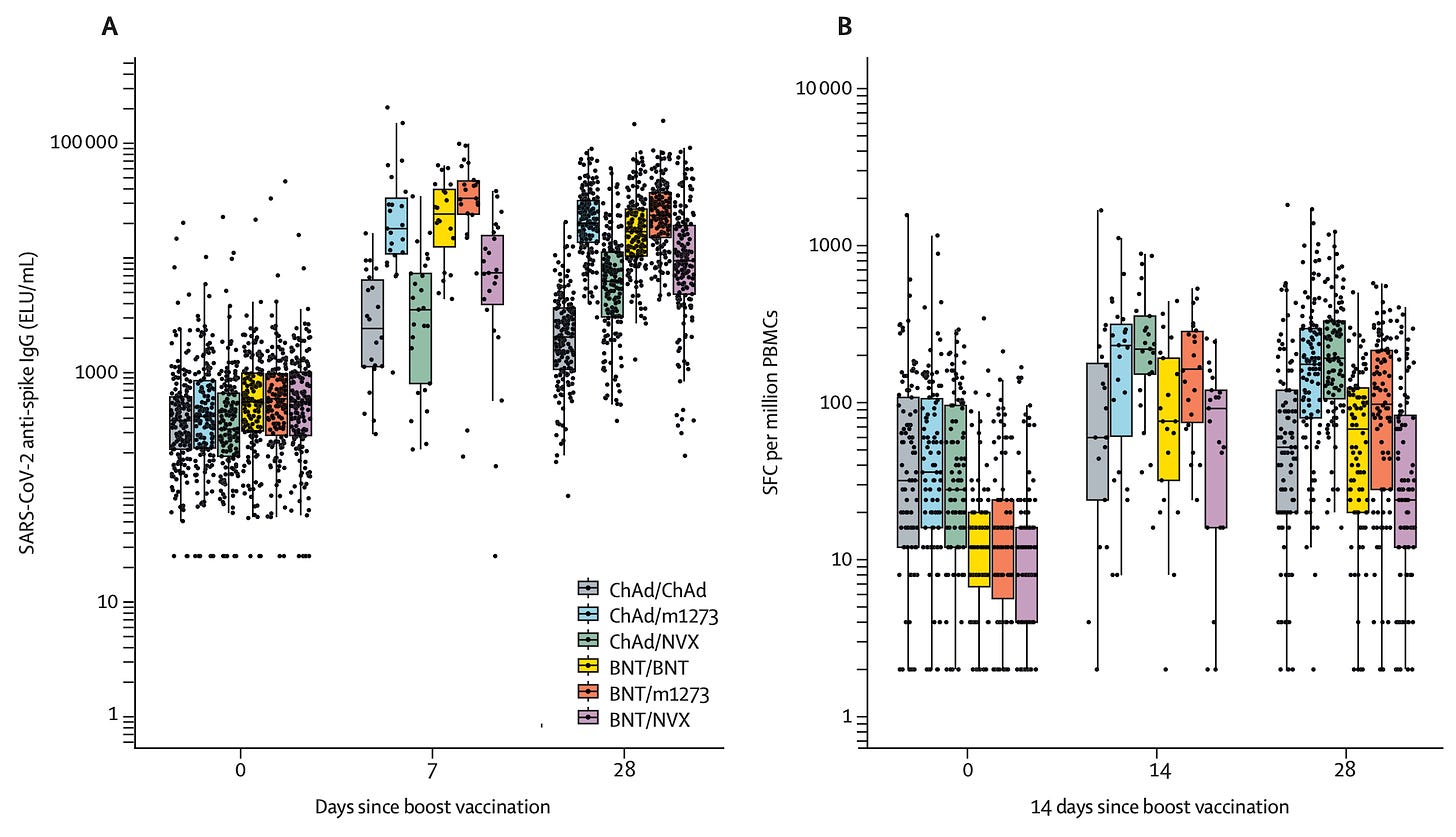
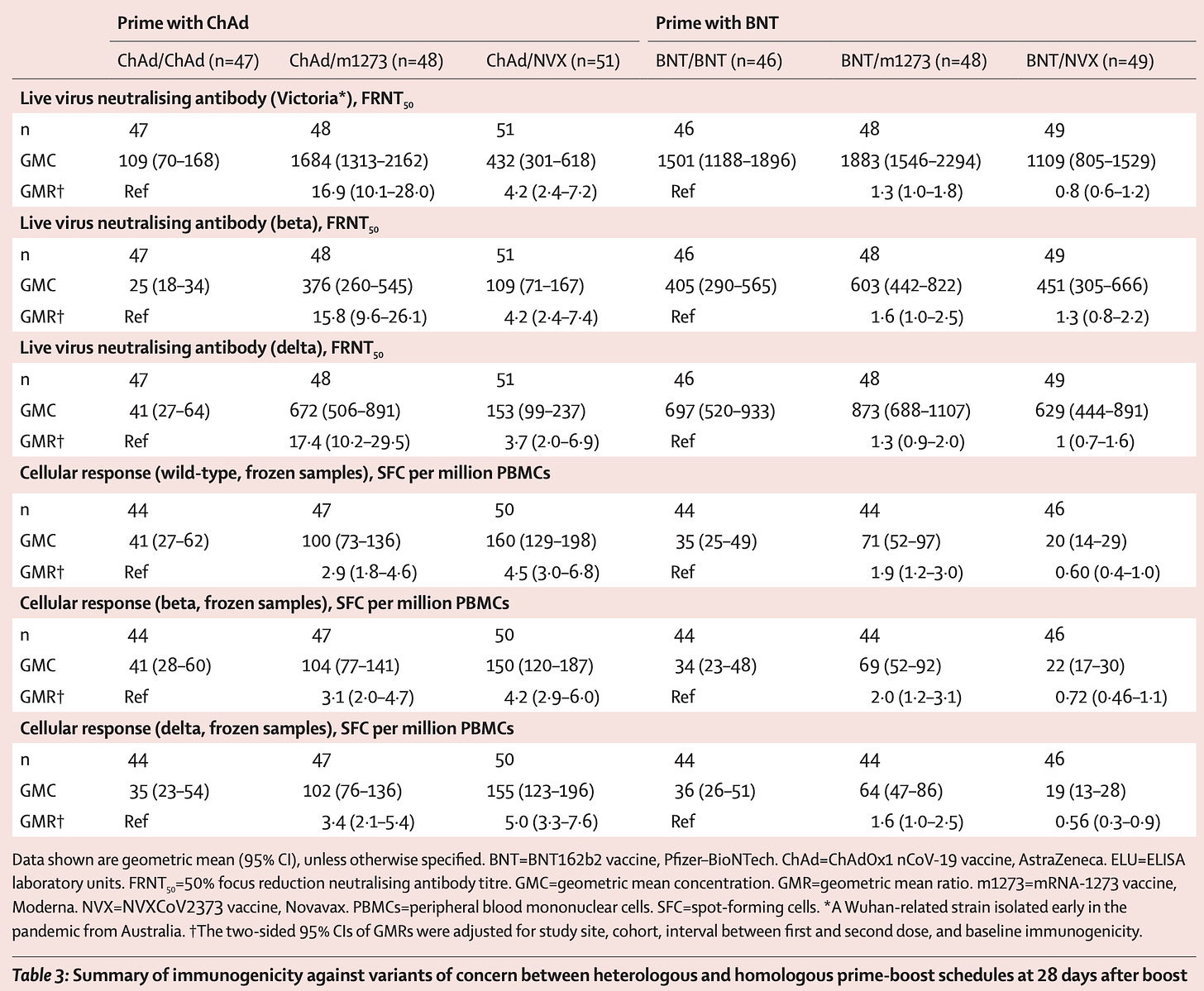
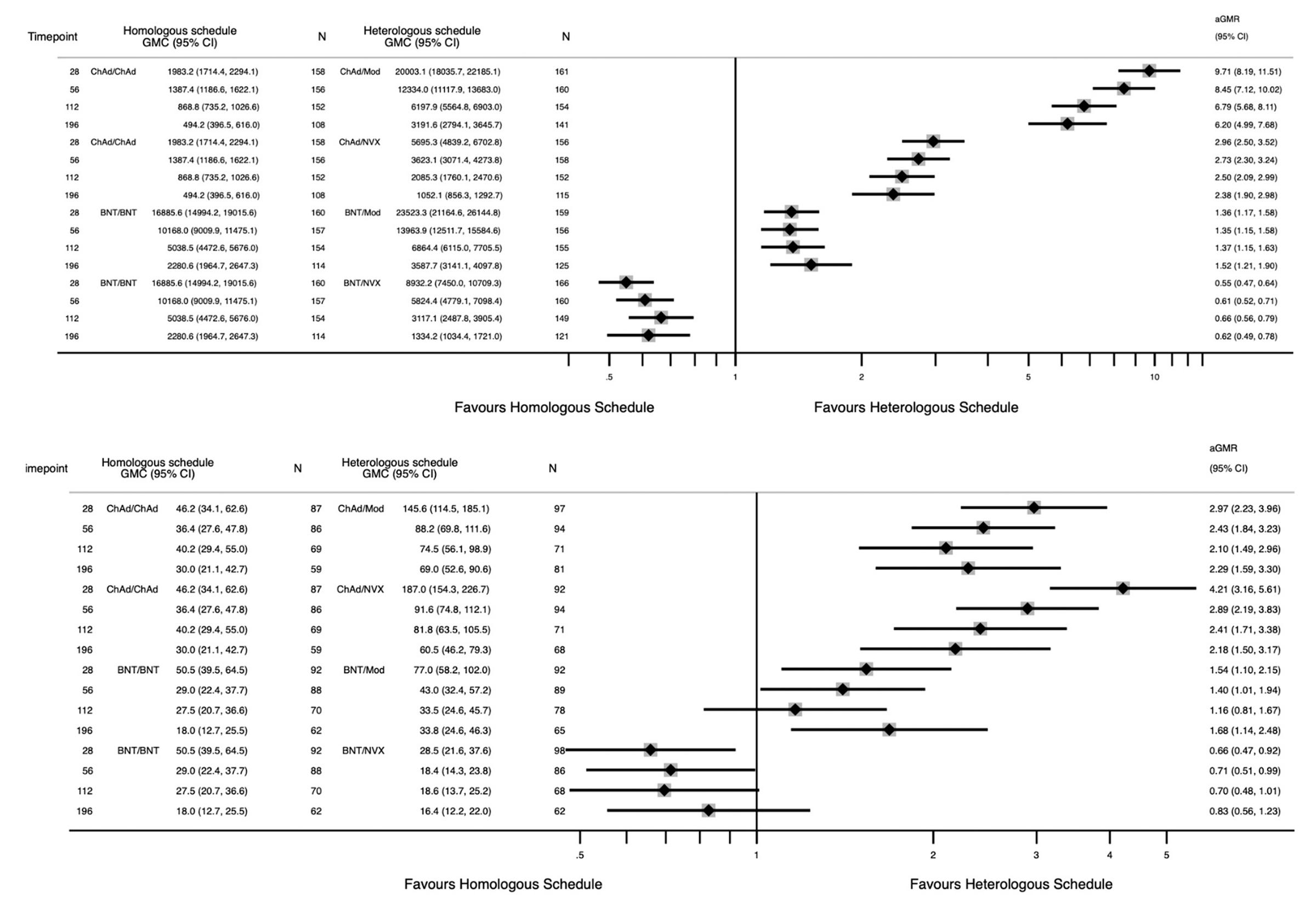
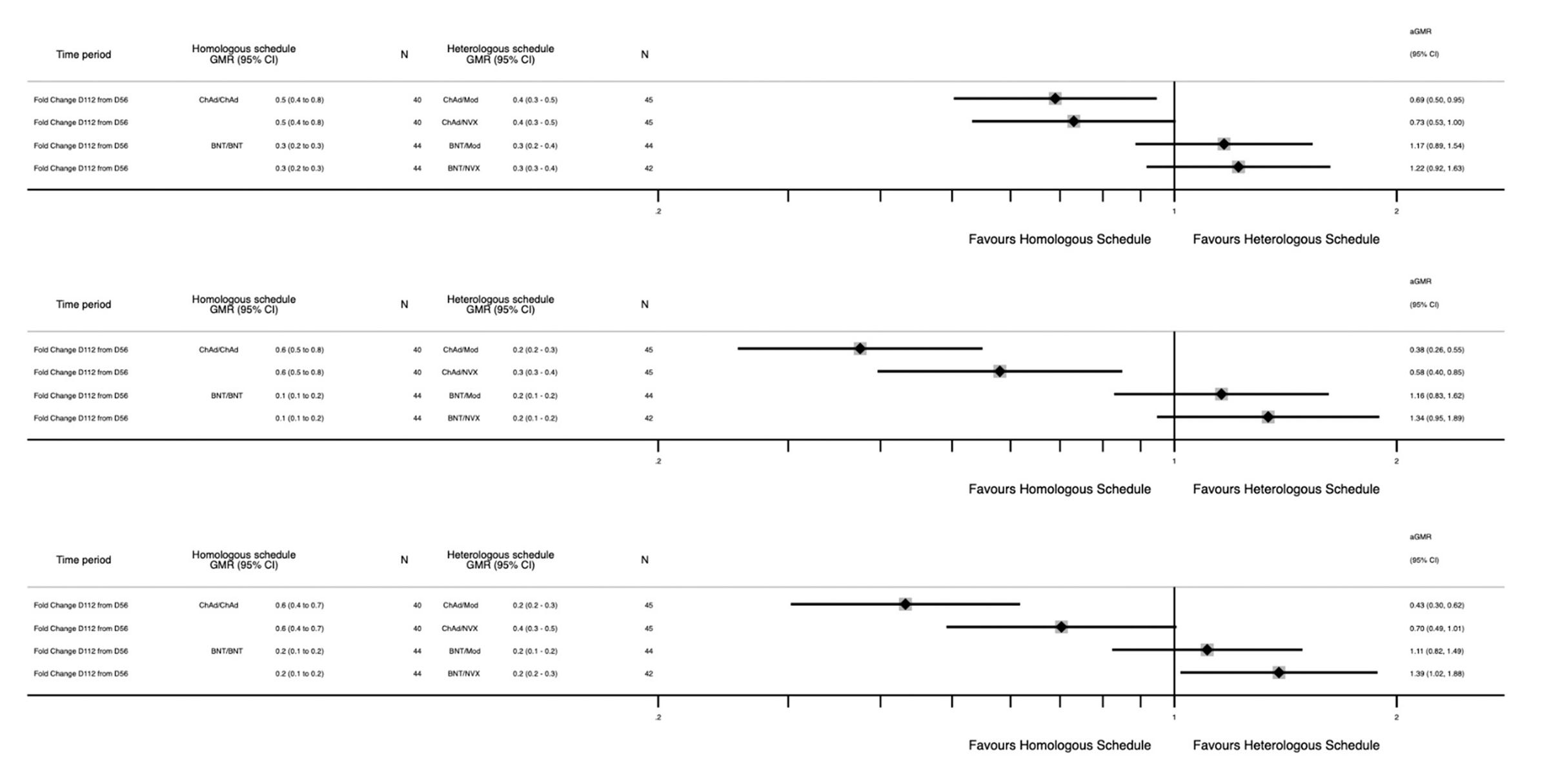


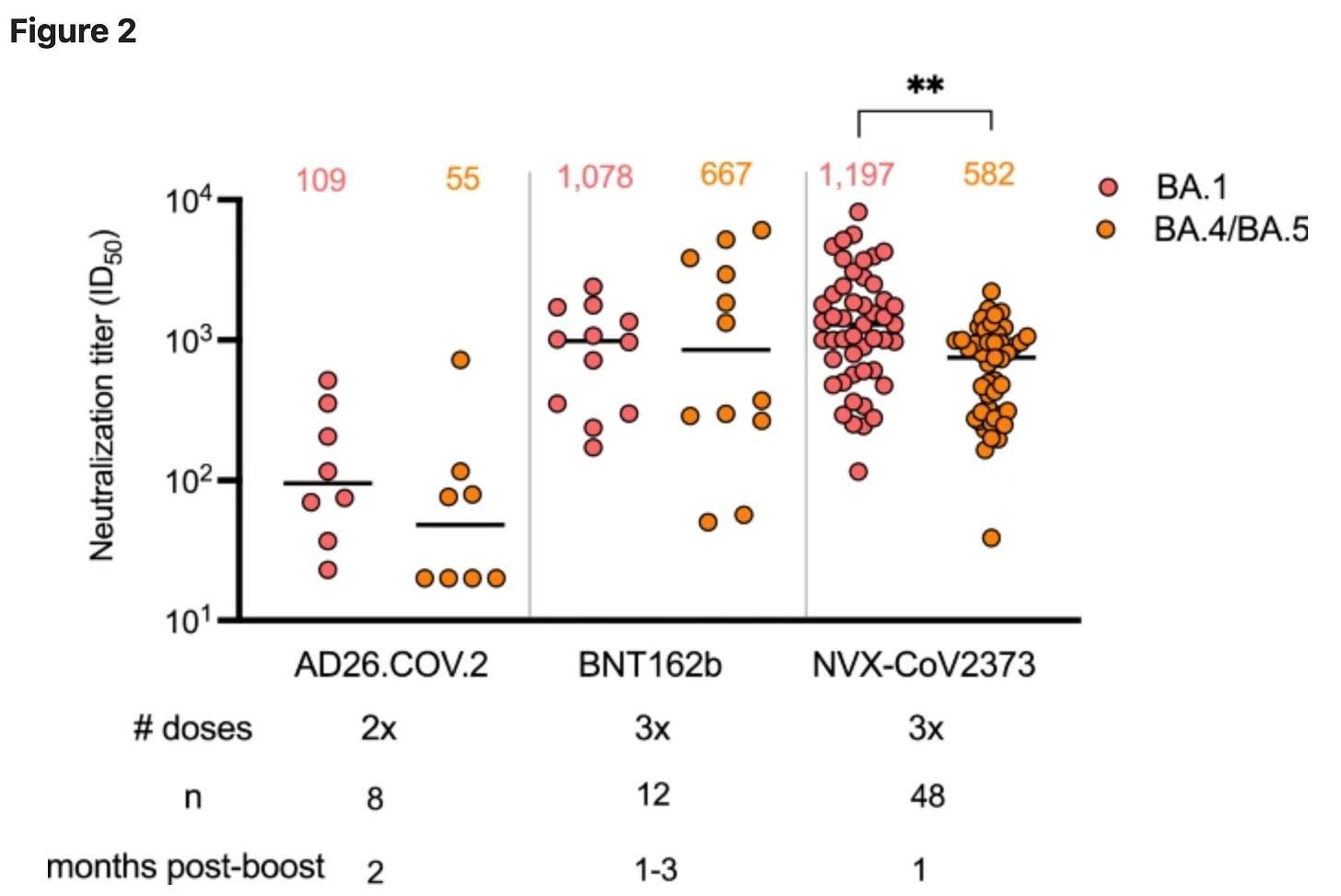
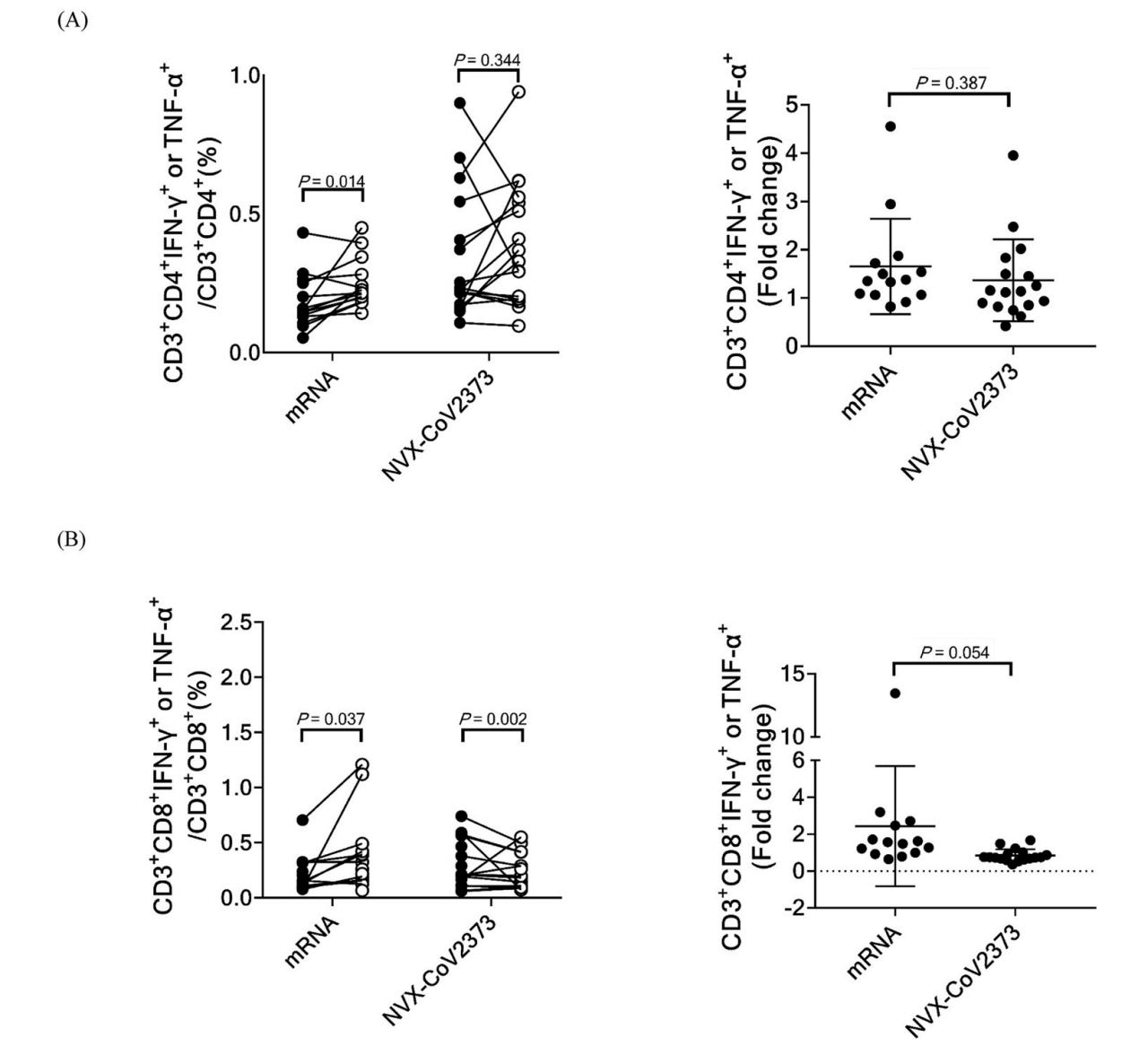
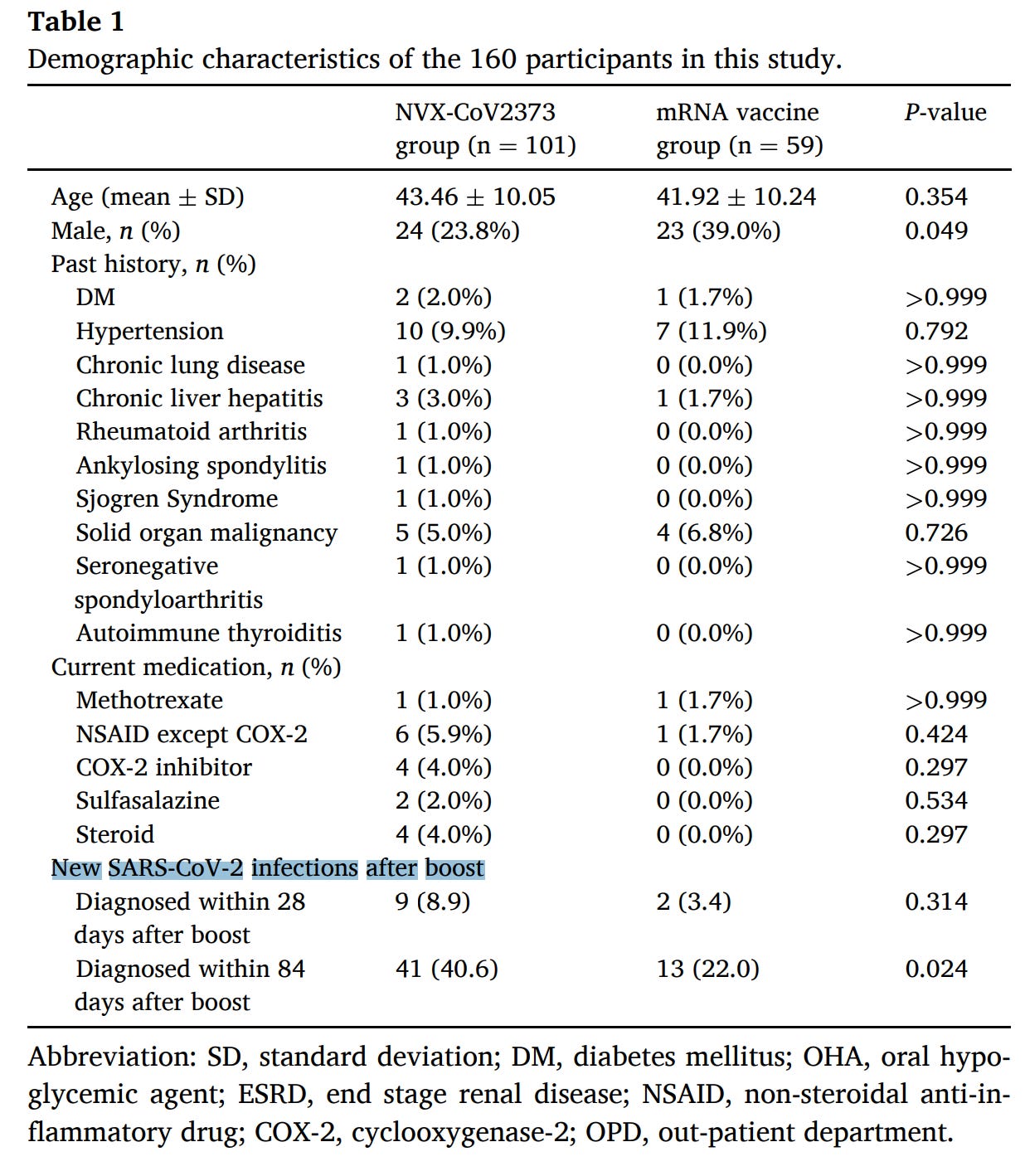
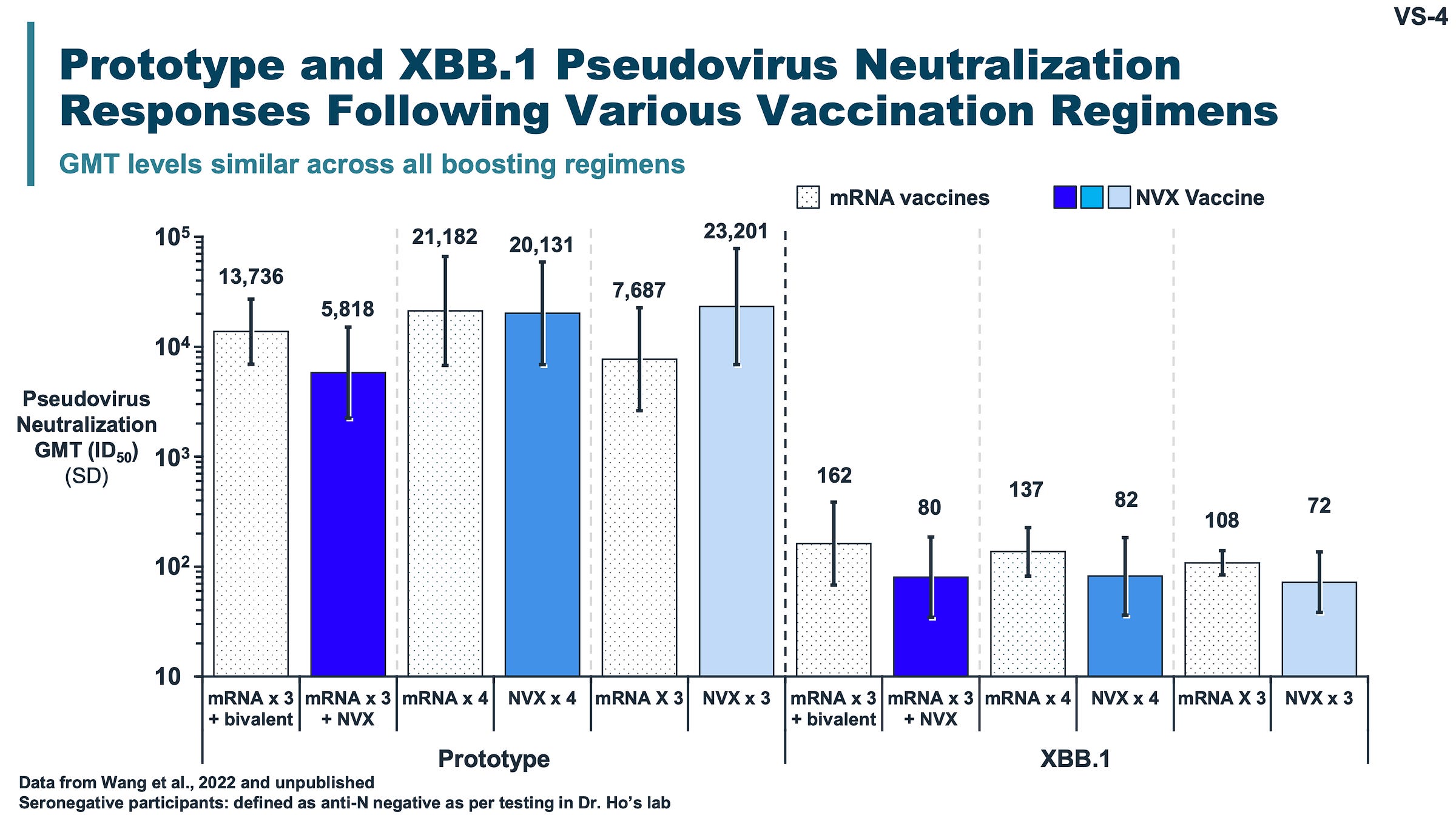

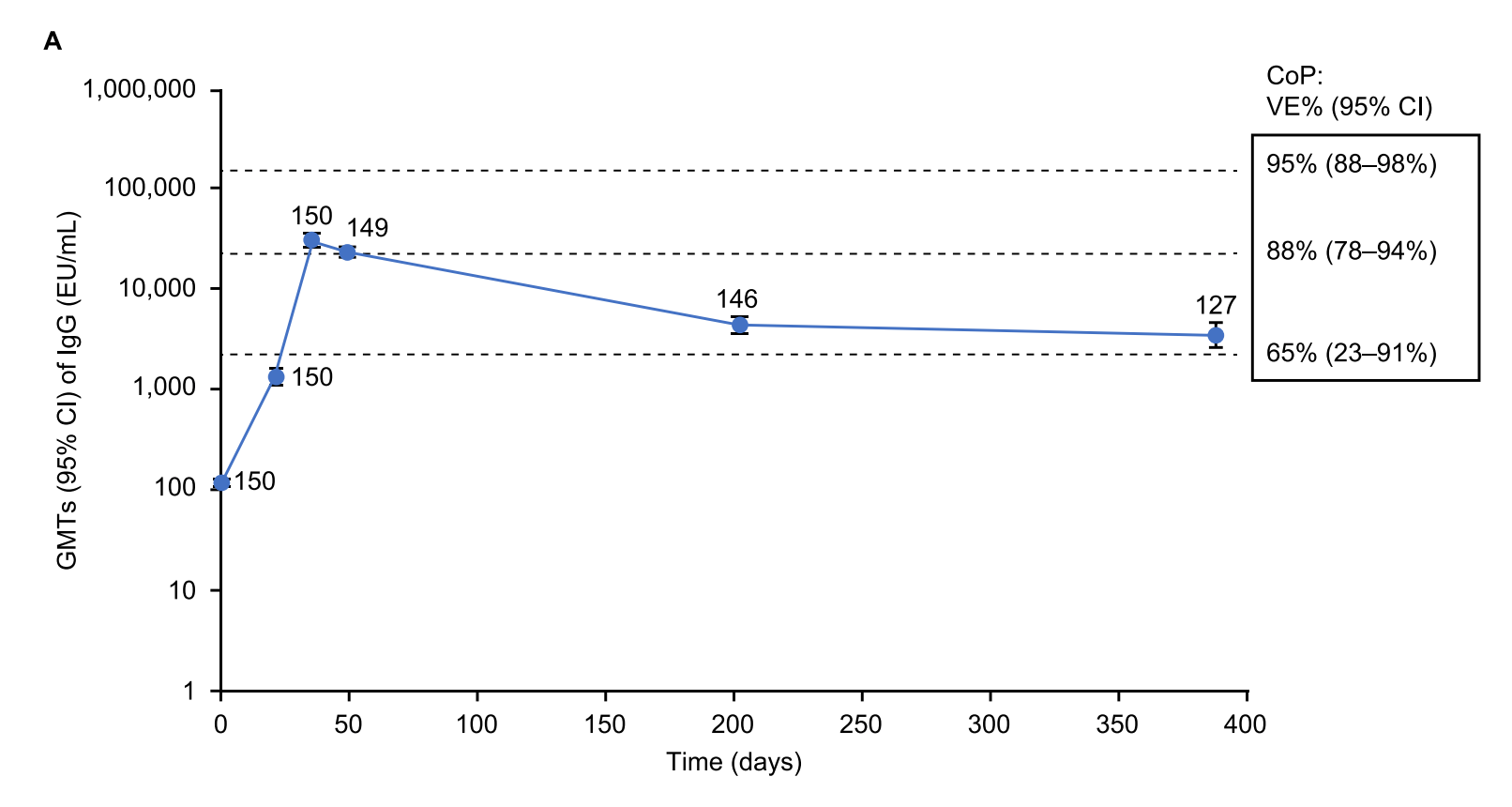
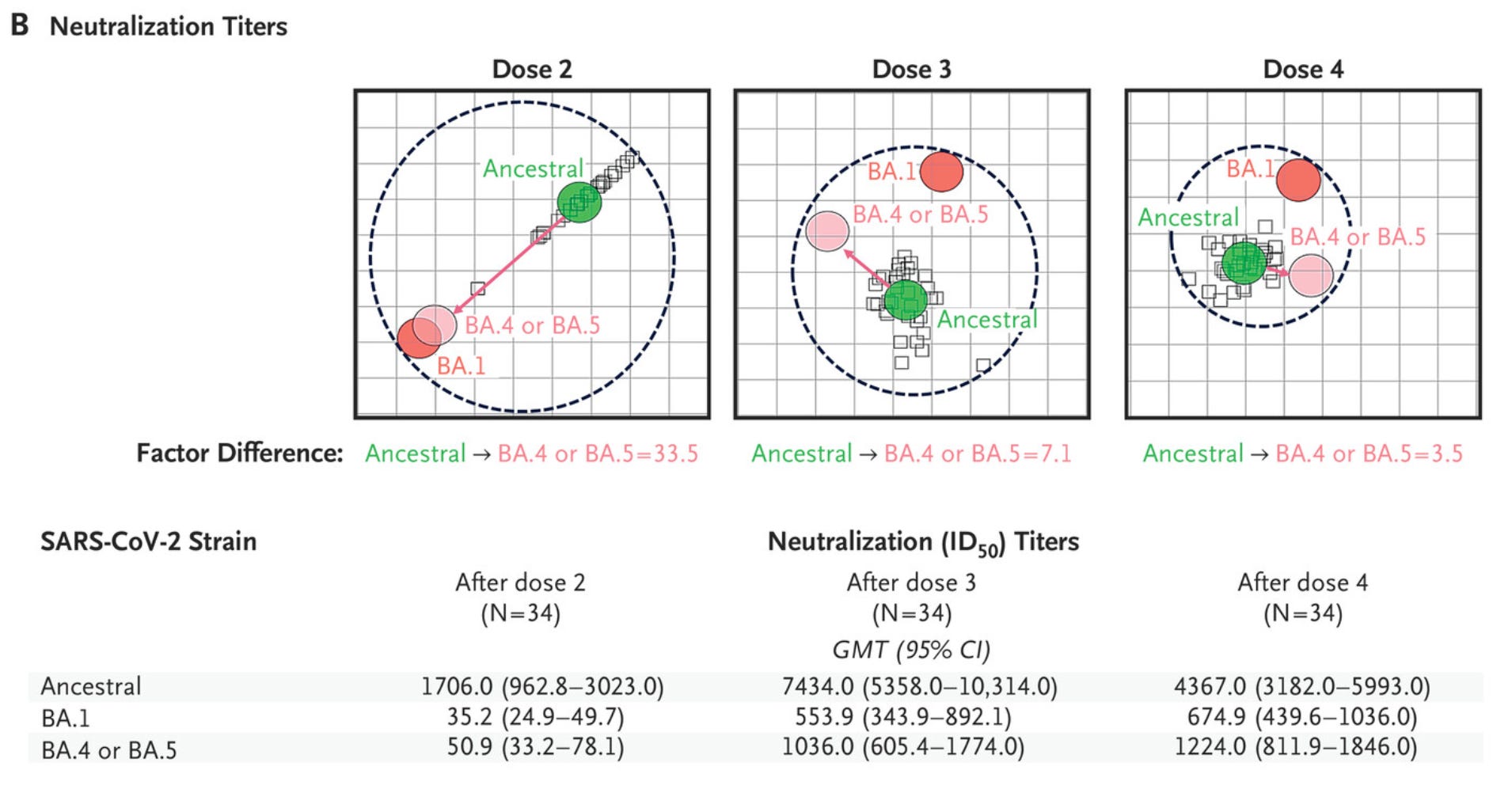
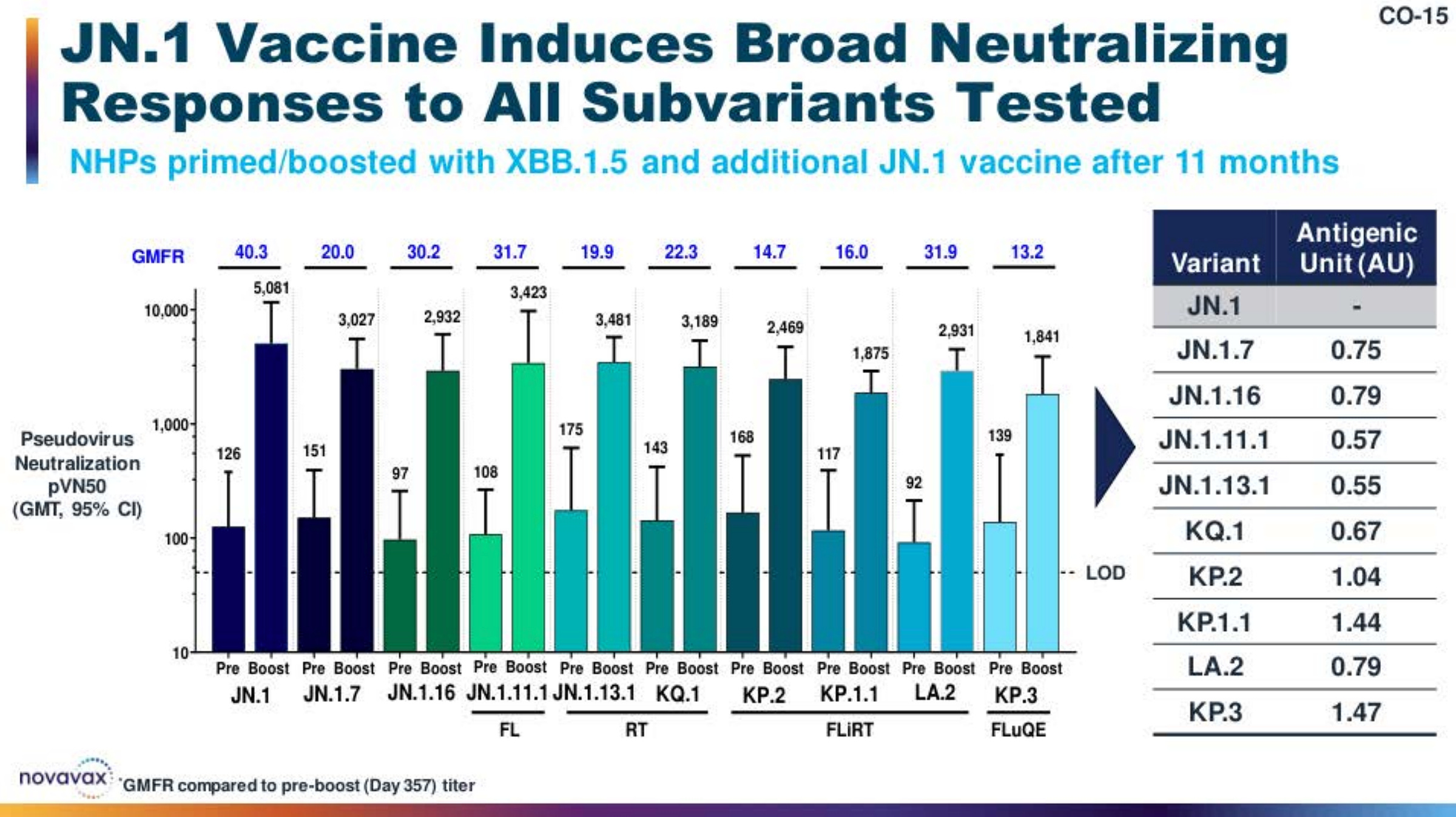

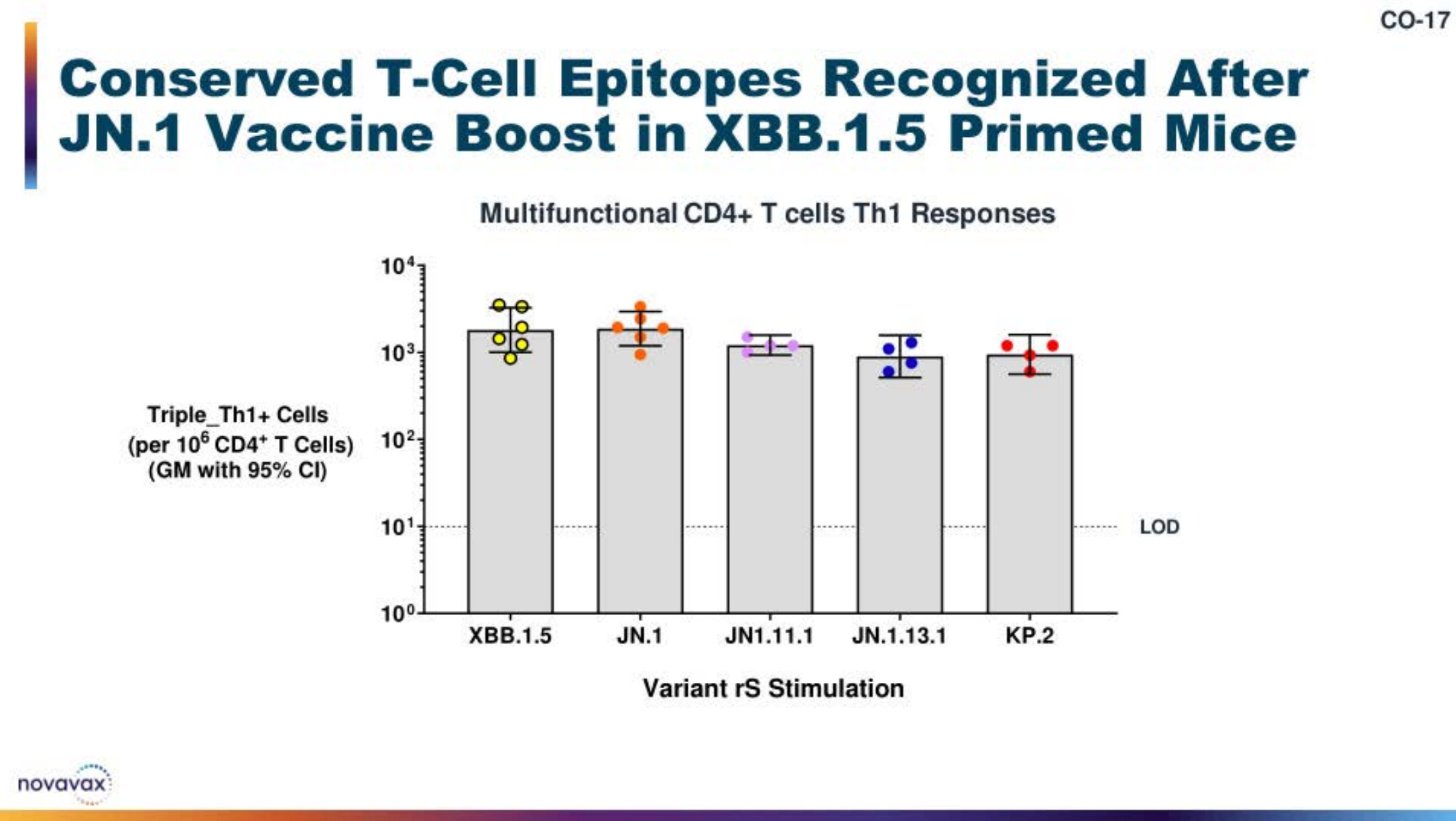
Whoa, thank you! This was one helluva deep dive, and I really appreciate all the time and effort you put into pulling all of this together.
The Novavax Fandom is quite vocal, and the folks insisting on sinister motives for it not being more available are exhausting.
I did get a Novavax XBB vaccine in January mostly because I wanted to check it out. I had previously taken every Moderna spork available, plus I had covid in August 2023, so I figured I was still decently protected against severe disease even if the Novavax wound up being a total dud. (I'm otherwise healthy, I still mask indoors, I'm a C-R box nerd, etc.)
I admit it was a pleasant surprise not having the usual post-mRNA blecchies, but given everything else you discussed (especially them citing horribly problematic supplement shills -- WTAF??) certainly gives me a lot of pause.
Thanks again-- I really enjoy reading your writing.
WHAT SHE SAID! That was one helluva read, and I went and slammed the subscribe button pretty hard and fast. I particularly appreciate FINALLY getting a bit of an explanation of how exactly the furin cleavage site worked, I've been looking for that for about four years. (This missing piece for me was that there was a fusion peptide held between S1 and S2.)
I admit to being a little bit of a Novavax stan (tho less so now) although I always always disclosed to people that my affection for it was not entirely logical. (Partly I have a very deep-seated belief in Diversity is a Good Thing, and want there to be alternatives to the mRNAs.) I myself rotate through All The Vaxes (again, because diversity) : AZ first (in part because I could get it fastest), then Moderna, Pfizer, and Novavax. Is heterologous the way to go? I don't know, but I'm not convinced that anybody knows. ;-)