For many people, looking at the components of a vaccine can spark confusion or concern. It’s hard to understand exactly why certain ingredients may be present just by looking at the list without significant specialized training, and one line of thinking argues that we need extensive data on each individual ingredient to be able to make a judgment of safety- but that’s not actually true. Here, I explain when it does make sense to consider vaccine ingredients individually and when it doesn’t (spoiler: it usually doesn’t).
Before I start discussing this, I want to make something clear: in writing this post, I am not prescriptively saying that you cannot look at individual vaccine ingredients, particularly if you have questions. You absolutely can. My point here is: when people have a big question, they work towards an answer by asking a series of smaller questions and getting the answers to those until a clear picture forms. But, sometimes, people with a big question may set up smaller questions that won’t help them answer that big question, even if it might seem like they can- and I see this happen a lot with questions about vaccine ingredients.
The big question that most people want to answer with vaccines is: “Is this vaccine safe?” But looking at the ingredients is one of those smaller questions that is generally not productive in answering that question (for reasons I will explain below). If it’s just a matter of curiosity, it can be looked at (I go through an example later), but if it’s in pursuit of answering that big question- that’s not the most effective approach.
Allergies- A Situation Where It DOES Make Sense to Think About Ingredients
The major case where you should think about vaccine ingredients would be allergies to a component of a vaccine (or, more precisely, allergies to a vaccine). But this has some nuance too. For example, egg allergies occur in 0.9% of all children in the US and some vaccines are made through culturing in eggs, including some (but not all) flu vaccines. If a vaccine would cause you to develop a serious allergic reaction (e.g., anaphylaxis) then the benefits of getting the vaccine are outweighed by the risks- but there’s some nuance to this particular example too.
The first one worth going into is that allergy testing is complex; most diagnoses of a food allergy can be made on the basis of a history and the presence of IgE antibodies against the food- both must occur. The most informative test is actually an oral food challenge, where, under the supervision of an allergist, you eat the food you are allergic to and are monitored for signs of an allergic reaction, which can then be treated immediately by the allergist and their team. The presence of IgG antibodies against foods does not indicate an allergy, and the presence of IgE antibodies alone (i.e., without a proven allergic reaction to the food) is not enough to conclude that an allergy exists. I raise these points because there is some predatory marketing of allergy tests which examine inappropriate biomarkers for allergy.
However, there are instances where people can be safely vaccinated with a vaccine that contains a component that they are allergic to (in fact, this is usually the case). For example, even though some flu vaccines are made in eggs and may contain small amounts of residual material from the culture medium, people with egg allergies can be safely vaccinated with these vaccines without additional precautions (but, for those who don’t want to take that risk, there are also flu vaccines made in cell culture that does not involve eggs). But there are some vaccines made in eggs that can pose a risk, such as the yellow fever vaccine. In the event that an allergy to a vaccine exists, the vaccine might be administered in graded doses in a controlled setting to manage any possible allergic reaction and desensitize from the allergy.
Gelatin allergy is a more important one because, while rare, people who have a history of immediate allergic reaction upon exposure to gelatin may experience allergic reactions from a gelatin-containing vaccine (this is not the case for any other vaccine ingredient). Gelatin-free alternative vaccines may exist in some cases. Gelatin may also be important in those who have alpha-Gal syndrome. An allergy to gelatin in general though does not automatically mean an allergy to a vaccine that contains gelatin.
So, what does this mean for what you should do when you go to get vaccinated? In general, inform about any allergies you may have and in particular allergic reactions you have had to vaccines. If you have had such experiences, consider discussing with a board-certified allergist regarding what options may exist for you so that you can safely be vaccinated.
Types of Vaccine Ingredients and Examples
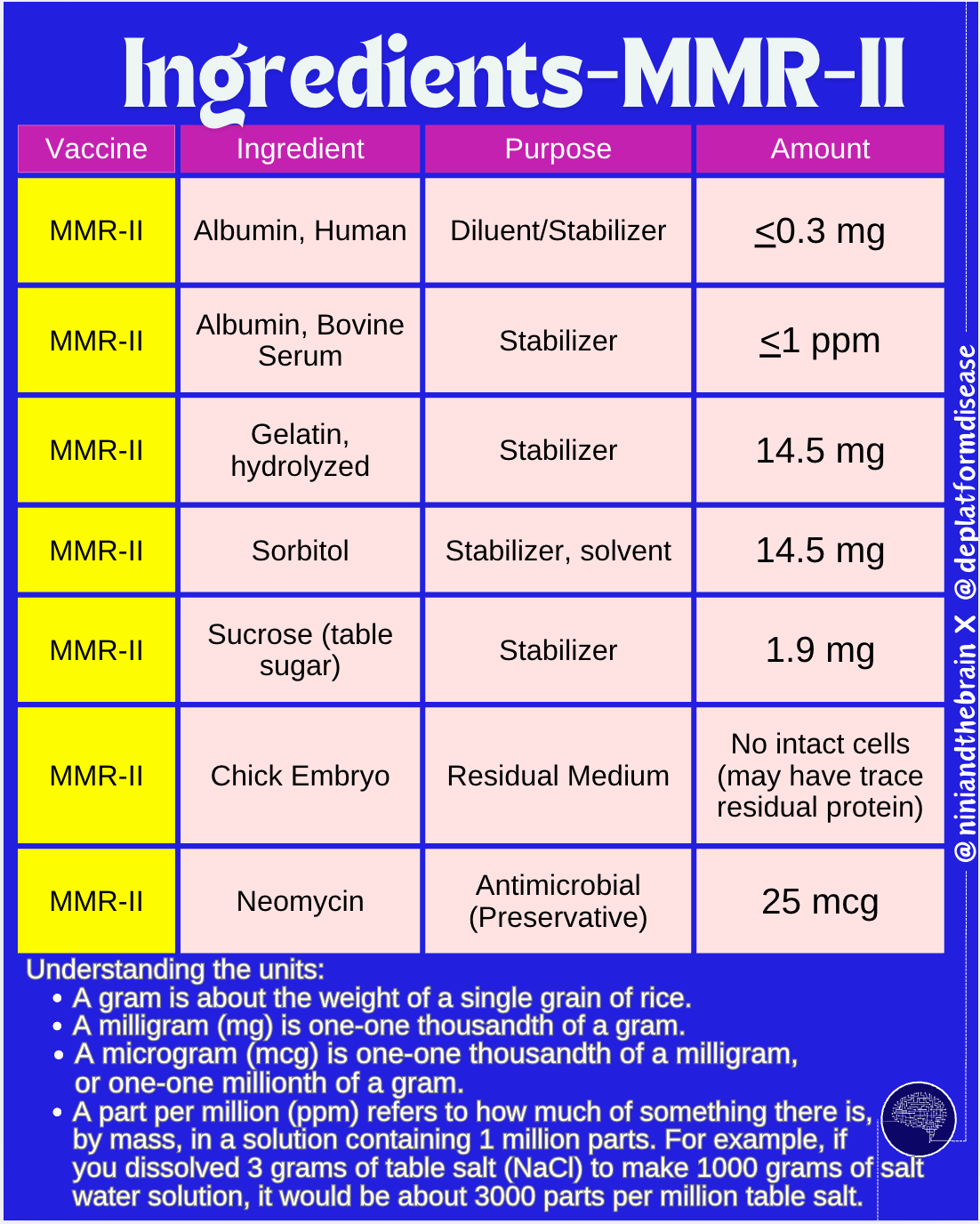
We should talk about the types of things that go into vaccines before we continue this discussion. Broadly, vaccine ingredients fall into a few classes: antigens, adjuvants, preservatives, stabilizers, manufacturing residuals, and inactivating agents. These are elaborated upon below.
Antigens (see chapter 1 of the Purple Book): These may also be known as immunogens (I prefer that term)1. They are the active ingredient of the vaccine- they are the thing that you are trying to stimulate an immune response against. By far, these are the most important component of vaccines. You may see these classified in several ways, but this is the organizational scheme I prefer:
Live attenuated microbes: Microbes (viruses, bacteria, parasites) that retain the ability to replicate and are alive, but are adapted to grow well in conditions that differ substantially from those in the human body (for example, they may have large gene deletions that are compensated for by the culturing medium but that cannot be compensated for within the human body). This means that their ability to replicate is very limited within the human body (though more antigen is generated inside the body once they are given) and they will almost never cause disease (the key exception is in cases where they are given to severely immunocompromised people, which is a contraindication for receipt of live attenuated vaccines). Live attenuated vaccines often give long-lived immunity (decades to life in some cases) with a single dose, but not always, and they are not a viable option for all infectious diseases. In some cases, live attenuated vaccines can mutate and regain the properties required for them to cause disease (such as with the oral polio vaccine). Examples of live attenuated vaccines given routinely in the US are: MMR (measles, mumps, rubella), varicella (chickenpox), MMRV (measles, mumps, rubella, varicella), rotavirus (given orally), and intranasal flu vaccines.
Whole inactivated microbes: these are microbes that have undergone treatments that render them completely incapable of replication (i.e., they are killed). There are many options by which you can achieve inactivation such as chemical (e.g., β-propiolactone) or radiation (e.g., ultraviolet light). Inactivated vaccines however may experience problems in that the process of inactivation can alter the structure of the antigen, which may interfere with the ability of the immune system to mount responses that match the actual microbe that circulates. In most cases, whole inactivated microbes do not give long-lived immunity with a single dose and require multiple doses. The inactivated polio vaccine is an example of a whole, inactivated vaccine.
Subunits: Instead of an entire microbe, these contain purified discrete components from the microbe that are important for the immune system to target to achieve protection (this is usually a protein from the microbe but it can be a sugar). However, these are generally incapable of inducing strong immune responses on their own and require adjuvants (see later). Several types of subunit vaccines exist. Toxoids are inactivated toxins- the toxin is important to the disease process so we want an immune response against it, but we don’t want any of the toxic effects from the toxin. Other types of subunit vaccines do not require inactivation. Polysaccharide vaccines are another example, using sugars from the microbe as a target, but today these are usually used as conjugate vaccines, where the sugar is linked to a protein carrier because this gives much better immune responses. Protein subunit vaccines can exist in a free-floating form (soluble) or as part of virus-like particles (essentially, a bunch of the proteins linked together in the arrangement they normally occur in on the virus or a similar one). Subunit vaccines are favored often because of their excellent safety record, but usually require multiple additional doses after the first to maintain immunity or to reach protective levels of immunity. Examples of subunit vaccines given routinely include the HPV vaccine, recombinant flu vaccines, DTap and Tdap (diphtheria, tetanus, acellular pertussis), RSV vaccines, pneumococcal vaccines, and meningococcal ACWY vaccines.
in vivo-expressed Antigens: the example people would be most familiar with here would be mRNA vaccines. In this case, the vaccine itself does not contain any of the antigen, but rather, the antigen is produced only once the vaccine has entered the cells. While this idea of the antigen being made inside the cells may sound new, it’s actually not- this is the major way that live attenuated vaccines make their immunogens (the live attenuated vaccines may replicate for a few cycles before being eliminated, but this still means most of the antigens will come from the replication process). These address many of the limitations of other types of vaccines: because no intact microbe is used, they are safe for immunocompromised people to take and there is no risk of a microbe reverting to a form that can cause disease. They require no additional adjuvant to work. They present immunogens in the context in which our immune system evolved to handle them (viruses work by making antigens within our own cells and our immune system evolved with this fact as a focal point of its strategies against viruses), which means that they can also produce immune responses that inactivated and subunit vaccines cannot effectively induce (i.e., killer T cell responses). The immunity elicited by them also appears to be very durable, but may require more than one dose.2 They can also be produced much more quickly than the other types of vaccines, which makes them ideal for responding to a public health emergency. The major downside with these (at least in the case of mRNA vaccines), is that they tend to be highly reactogenic, meaning they frequently induce significant symptoms as a consequence of the immune response (fever, injection site pain, redness, swelling near the injection site, joint aches, etc.), which, while not unsafe, can be disruptive to daily life for a brief period.
Adjuvants: substances added to the vaccine to enhance or enable an immune response to occur. Not all vaccines require adjuvants, and specific adjuvants are generally not interchangeable. Throughout history many different adjuvants have been used, but for most of the history of vaccines when adjuvants were used, aluminum salts were the only ones allowed for human use for about 70 years. Today, many additional adjuvants have demonstrated safety and effectiveness for human use (they don’t really have catchy names though) such as AS01, AS03, AS04, MF59, Matrix-M, CpG-1018, Alhydroxyquim-II and others. In addition to ensuring that a protective immune response develops (or that one occurs at all), adjuvants help steer the immune response to ensure that the correct type of response develops (for example, the immune response you need to deal with a worm is not the same as what would be useful for a virus), and they allow for dose-sparing of the antigen. On their own, adjuvants do not protect against infectious diseases (some possible exceptions but the effect is still much weaker than if incorporated with an antigen). We also know that some vaccines will not work without the inclusion of the correct adjuvant.
Preservatives: These are substances that are included to prevent microbial growth. For example, if a vaccine comes from a multidose vial, each time the needle passes through the vial stopper, bacteria may be introduced and grow. Such vaccines require preservatives; without them, people can develop skin abscesses and even fatal sepsis. Preservatives may also be included in the process of manufacturing a vaccine to prevent microbial growth. Over time, advances in the technologies used for vaccine production have greatly reduced the need for preservatives, and not all vaccines require preservatives (for example, mRNA vaccines contain no preservatives- but they have to be used within 6 hours of thawing as a result). Thimerosal is a famous preservative that gained a lot of attention for entirely the wrong reasons and has a complex and amazing history. It has been removed (but didn’t really need to be) from childhood vaccines other than multidose flu vaccines (the overwhelming majority of available flu vaccines do not contain thimerosal and you can request a thimerosal-free vaccine if that matters to you) and it is used in the production of but then removed from one DTaP and one DTaP-Hib vaccine (but it may still be present in trace quantities). Phenoxyethanol is another preservative which may be used in the inactivated polio vaccine.
Stabilizers: Storage of vaccines before their use may involve a freeze-drying process and cycles of freezing and thawing. Stabilizers help to ensure that these processes do not damage the vaccine (thus compromising its ability to elicit protection). Gelatin is an example of a stabilizer. Other stabilizers include sugars (glucose, lactose, sucrose), amino acids, hydrolyzed casein (a milk protein), and albumin. Stabilizers also include buffers- substances added to the vaccine to help maintain a constant pH. Usually these are salts such as sodium phosphate, tromethamine, or amino acids.
Manufacturing residuals: The process of manufacturing vaccines often involves the use of cells to grow the antigens. Once made, the antigens are purified out. However, minute quantities of culture residuals may remain. These can include things like antibiotics (such as neomycin- found in neosporin), trace quantities of DNA from the culture medium3, trace quantities of proteins other than those used for the vaccine (like egg proteins for vaccines manufactured in eggs), and other substances added to the culture medium important during the manufacturing stages. These are all excipients which are purified out during the production process before the final vaccine product is made. Nonetheless, no purification process is 100%, so trace quantities of these may remain.
Inactivating agents: for vaccines that require antigens to undergo chemical inactivation, after purification, there may still be some trace quantities of the inactivating agent present in the final product. A common example of an inactivating agent is formaldehyde, used in the production of tetanus, diphtheria, pertussis, and polio vaccines (note that formaldehyde is also a natural byproduct of our metabolism and a typical 2-month old infant’s blood contains 1100 times more formaldehyde than what is present in any vaccine).
The Big Fallacy: Long-Term Studies of Every Ingredient
There’s a line of reasoning that goes something like this: unless we have detailed long-term safety data on every component of a vaccine individually, we cannot honestly claim that the vaccines are safe.
To illustrate why this doesn’t make sense, consider the following example:
Acetaminophen (Tylenol) is a major cause of liver failure throughout the US, resulting from overdoses of the medication. Acetaminophen causes liver injury by depleting the cells of glutathione via a metabolite of the drug called NAPQI, which makes them susceptible to oxidative stress that ultimately kills liver cells. Management of toxicity relies on the use of N-acetylcysteine (NAC), a precursor to glutathione, to restore the normal internal environment of liver cells.
Now, suppose that we live in a world where neither acetaminophen nor N-acetylcysteine are approved medications for humans and we have no data on how these work individually. I make a pill that contains NAC and acetaminophen together and I want to market it for use as a pain reliever, so I initiate clinical trials, part of which involve a repeat dose toxicity and dose-escalation study. Knowing what we know today, it’s unlikely that any clinical trial participant receiving this formulation would show signs of liver injury. But let’s say I studied the acetaminophen and N-acetylcysteine individually and did these same studies. I would find that the acetaminophen could cause significant liver injury at higher doses.
But does that matter? If I want to market the acetaminophen as a drug- absolutely. But I’m not trying to do that- I’m interested in my combined acetaminophen-NAC pill.
How do I use the information from the acetaminophen toxicology data to help determine whether or not my acetaminophen-NAC pill is safe?
Or how about this scenario:
An organ transplant patient is infected by SARS-CoV-2. Given their very high risk for a poor outcome, a course of Paxlovid (nirmatrelvir/ritonavir) is immediately initiated. The patient is subsequently hospitalized with tacrolimus (an immunosuppressive medication used to prevent organ rejection) toxicity manifest by acute kidney injury.
What happened?
Nirmatrelvir is the component of Paxlovid that produces an antiviral effect, but it is eliminated too quickly to be usable on its own. To address this, ritonavir is added to slow down the metabolism and elimination of nirmatrelvir, so that it can be maintained at therapeutic concentrations.
But ritonavir does not solely affect the metabolism of nirmatrelvir- it targets a protein called CYP3A4, which is the protein most commonly involved in drug metabolism, including for tacrolimus. Tacrolimus is inactivated by CYP3A4- but now we’ve inhibited it with ritonavir. That causes tacrolimus levels to rise to the point of becoming toxic, causing kidney injury.
Tacrolimus, nirmatrelvir, and ritonavir are all perfectly safe at their indicated doses when examined individually- but when you look at the combination, a novel toxicity risk arises.
The fact is: when we are interested in the safety of a medication, the relevant thing to test is: the entire medication. Looking at individual components only can both show additional risks not present in the combination or fail to reveal risks that arise only with the combination of all of the components. That’s why when we want to know about vaccine safety, we focus study resources on seeing how the entire vaccine affects people, rather than separating out each component and only after each one has proved safe do we decide to have the trial of the entire thing.
The practical issues: aluminum salts as a case study
One of the vaccine ingredients that often gives people pause is aluminum salt adjuvants, which I have thoroughly discussed here. Let’s say now we go to our demand for a long-term study in humans on the effects of aluminum salt adjuvants in isolation given by intramuscular injection (as is done with vaccines). What’s the problem with this? Well, as I hope I’ve illustrated to this point, this doesn’t actually get at whether or not vaccines containing aluminum salt adjuvants are safe or not (which is the question we actually care about).
But there’s actually an even bigger issue: this study is unethical. Why?
There are 8 basic considerations for determining whether a clinical trial is ethical (it does get more complex than this but this is a useful starting point to examine whether or not a study is ethical):

There are four key problems with such a study of aluminum adjuvants:
Favorable risk-benefit ratio: Aluminum adjuvants when injected frequently cause local reactions, which are mild, and they also require an intramuscular injection to administer, which poses minimal but nonzero risks. However, aluminum adjuvants without the context of a vaccine antigen do not plausibly have a chance of benefiting the clinical trial participants (unless we get data that says otherwise). Think about this: if you believe that this study is important and missing, would you be willing to volunteer your child for it? If not, why is it fair to expect that other people volunteer their children for it? This gets even more fraught when you add in the consideration that the participants for such a study would have to be infants and potentially even neonates, because children are part of the class of vulnerable populations that demand additional safeguards in clinical research. We CAN (and have) done these studies on animals, but we need to be mindful that animal physiology is not a perfect reflection of human physiology, and we are not yet at the point where other types of models (e.g., organoids) can comprehensively inform about toxicology.
Lack of scientific validity as proposed: This study is trying to get at whether or not the aluminum adjuvant in a given vaccine (or perhaps cumulatively over a vaccine schedule) is safe. When we already have data evaluating the entire vaccine, and in the context of the entire vaccination schedule, this study is lacking in merit because it is not going to answer the question we care about. Now, if, hypothetically, we discovered that aluminum salt adjuvants on their own could have some kind of benefit in the demographic of interest based on well-founded science, then we would be justified in performing this kind of study- but until this is the case, we cannot do it.
Edit (this point was made independently by both Dr. Elizabeth Stephenson and Druv Bhagavan in my conversations on the ethics of this sort of study with each of them; I initially omitted it because I was concerned that making the point could appear paternalistic and dismissive, but insofar as I am offering an analysis of the ethics here, it should be noted regardless as it would be a real consideration on the part of an Institutional Review Board tasked with determining whether such a study should go ahead)
Equipoise: To justify performing a clinical trial of this nature, there must be genuine uncertainty among qualified experts as to what the outcome of such a study would be (this is known as equipoise). Because as it is children are exposed to these levels of aluminum salt adjuvants through the vaccination schedule, and we have nearly 100 years of data on the use of these vaccines without evidence of a toxic effect from the adjuvants, it is difficult to argue that the condition of equipoise could be met. Now, as discussed earlier, the possibility of subadditive (i.e., the other vaccine components reduce the toxicity of the aluminum salts- like the NAC-acetaminophen example) does exist in principle, but with all of the other practical issues that arise, it is not compelling enough to justify a clinical trial.There is also another practical issue that arises: this study would need to attempt to tightly control exposure to aluminum adjuvants, and because they would be examined individually, this suggests that you would have to withhold aluminum salt-adjuvanted vaccines from the clinical trial participants. That is a categorical non-starter. You cannot leave these individuals at risk for very serious vaccine-preventable diseases simply to satisfy your own curiosities. You cannot withhold the standard of care.
For the sake of completeness, let’s go through how we can know the aluminum content in vaccines is safe. If you go through the entire current recommended childhood vaccination schedule, children may receive 1.58 to 3.18 milligrams of aluminum (as part of aluminum salts) in the first 6 months of life4. This is a very small amount of anything- but small amounts of some substances can still be toxic. Does this amount of aluminum pose a toxicity risk?
The first thing we need to consider is the form of aluminum we’re talking about. When we are thinking about toxicity from aluminum, we are concerned with toxicity due to the Al3+ ion. The major issue with this chemical species is that it is very reactive, and because of this can form reactive oxygen species that cause damage to cells. The aluminum in vaccines is delivered as part of a salt, meaning that Al3+ cannot be released until the salt is dissolved. However, aluminum salts in general have very poor solubility (for aluminum phosphate 0.000000022 g of Al3+ can be liberated per liter of water5). If that weren’t enough, we also know that the adjuvants used in vaccines have their minimal pH attained around physiological pH (7.4):


What does this mean practically? Well, because the vaccines using aluminum salts are given by injection, eventually all of the aluminum will end up absorbed- but the release of the Al3+ ion is going to be very slow because so little of it can be dissolved at any given instant in time (eventually, as the Al3+ ions are cleared, more will be dissolved until equilibrium is re-established, per LeChatelier’s principle). We aren’t going to be able to get anywhere near the levels of aluminum required for toxicity- but how do we know that?
Al3+ ions will readily complex with phosphate ions, and at one point these were used in dialysis patients and for preterm infants, in both cases delivered intravenously (i.e., 100% absorption instantly). This resulted in a condition called dialysis dementia or dialysis encephalopathy. You should notice something important though: all of these patients were on dialysis- they had minimal kidney function. Kidneys, in fact, are the major way that aluminum gets eliminated. Once in the bloodstream, it has a half-life of about 1 day. But that assumes normal kidney function.
This tells us that if kidney function is severely impaired, aluminum toxicity might be possible. But people who have impaired kidney function are actually recommended to get more vaccines than the average person because they are at greater risk for severe infections. Strangely (but not really), since the levels of aluminum in dialysate fluid have been restricted, dialysis encephalopathy has essentially disappeared, and even elevated levels of aluminum in the blood of dialysis patients without evidence of toxicity is much rarer- despite the recommendation for additional exposure to aluminum salt adjuvanted vaccines.

This would imply that vaccines are not a significant contributor to overall aluminum burden. In fact, aluminum is the third-most common element in the earth’s crust. Some antacids have 208 mg Al/tablet. Exposure to Al occurs through air, diet, cosmetics, food packaging, water, and industrial sources. Is this aluminum having a negative effect on people's health?
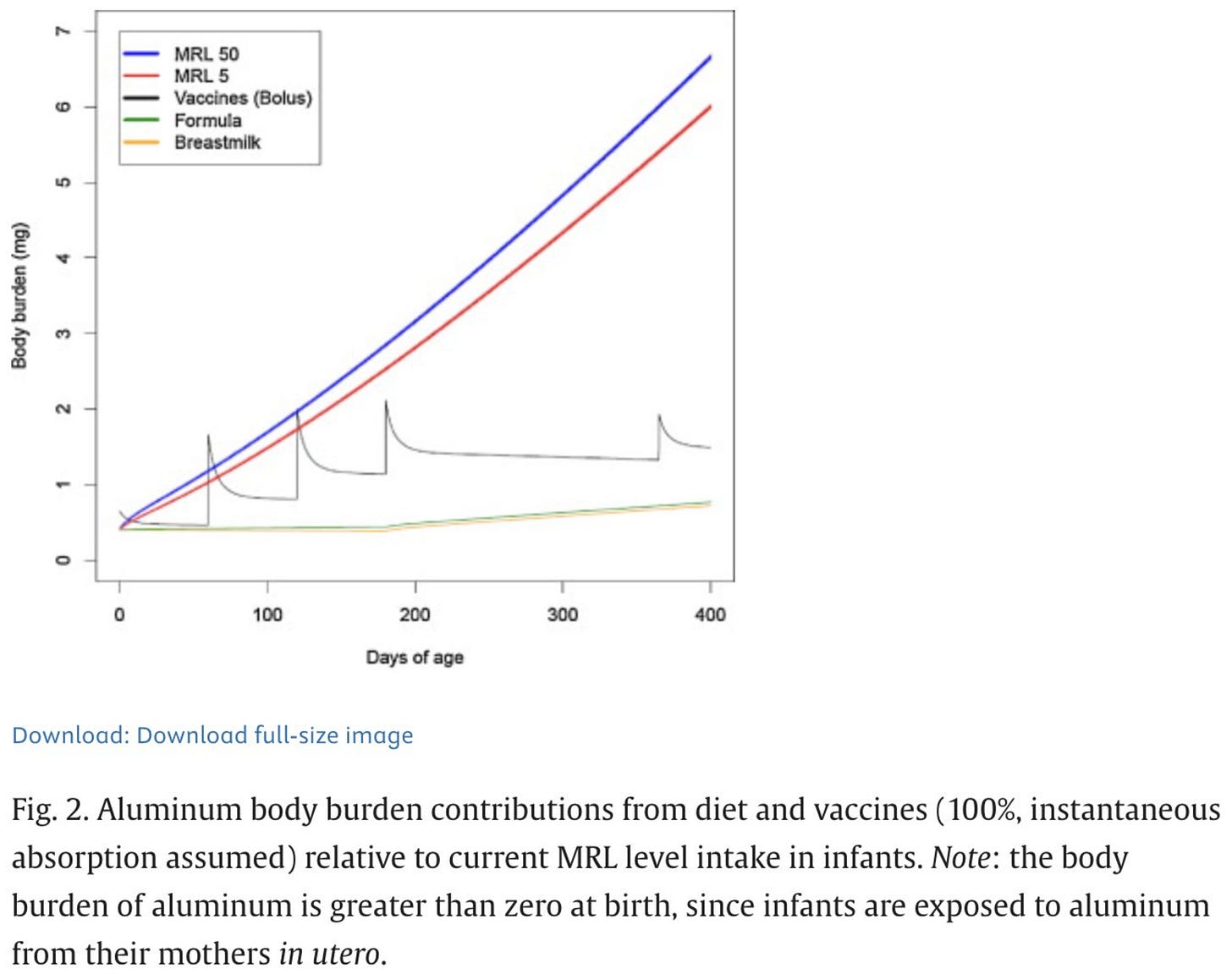
The data are very clear that they don't. If you assume 100% absorption of aluminum from vaccines immediately and compare this to the minimal risk levels (MRL) of aluminum (highly conservative estimates on what would be needed to start seeing toxic effects) you get this result:
But as discussed, even though there is a transient period where we exceed the MRL due to vaccines, this is based on a false assumption (that all of the aluminum is instantly absorbed into circulation). Still, this might not matter because because MRLs are set so conservatively that levels slightly above the MRL are likely safe as well. However, if you model it based on more realistic toxicokinetic data you get this:
Now, we are nowhere near the MRL. Furthermore, the body burden of aluminum from vaccines is still less than twice that from the diet.
That was a decent amount of work though- we could have just consulted the extensive body of data on aluminum adjuvanted vaccine safety and seen that nothing resembling aluminum toxicity has been reported (I checked).
But couldn’t these studies increase vaccine confidence?
From a consequentialist perspective, you might be able to judge these studies ethical with the rationale that the incredible social value of enhancing vaccine confidence justifies the risks to the participants so long as all other conditions are followed. But is this the case? Would these studies move the needle?
The disappointing answer is: probably not in any meaningful way. Let’s look at some history. Since Andrew Wakefield’s fraud in 1998 alleging a link between the MMR vaccine and autism, a flurry of very large, well-designed epidemiological studies were initiated to see whether or not this could be true. The evidence is overwhelming and clear: there is no link between the MMR vaccine and autism. What happened next? It was claimed that thimerosal was actually the part of the vaccine that was responsible for autism (the MMR vaccine has never contained thimerosal). Studies found no link there either. However, out of an abundance of caution (that later proved to be unnecessary) the FDA got rid of thimerosal from the vaccine supply (with the exception of multidose flu vaccines)- and the incidence of autism continued to increase. At that point some people decided that the actual villain of this story was aluminum- even though to that point overwhelming data on aluminum salt-adjuvanted vaccines showed no link to aluminum salt-adjuvanted vaccines and autism. Then it had to be the number of doses of vaccines kids were getting. Or maybe it was the combination vaccines. On and on it went, ignoring the same basic fact: vaccines do not cause autism.
While all of this was happening, a bunch of data was coming out looking at what could be driving the increase in autism- and pretty quickly the answer became very clear: changes in screening practices and diagnostic criteria. We also found through epidemiological studies that the overwhelming majority of the risk of developing autism could be explained by genetic factors, and we have learned that the processes that result in autism are initiated in utero before any vaccines are given. We have overwhelming data proving that there is no link between vaccines and autism no matter how you slice it- but instead, claims shifted to ignore this, villainizing specific ingredients and ignoring the actual known factors that are driving an apparent increase in autism. In short: the conclusion of those who felt a link between vaccines and autism existed was decided without consulting the evidence. When the evidence emerged that this was an incorrect position, new evidence needed to be generated to align with the conclusion. This is known as policy-based evidence making and it is the opposite of the scientific method, which begins with evidence to formulate a conclusion.
And while all of this was happening, autism and autistic people were constantly being dehumanized, with some seriously believing that measles would be a better fate for their child than autism.
Now, is there someone vaccine-hesitant out there who might be reassured by studies finding no meaningful risk from the individual ingredients of vaccines? Sure, I feel confident that at least one person out there like that does exist. But reasonable people thinking rationally about vaccination can come to understand this line of reasoning and see that testing each component of vaccines individually in the manner suggested does not make sense. The problem is, so often, we don’t approach these questions as purely rational actors (nor anything, for that matter), and sometimes that can be a good thing (depending on what people mean by “rational”).
Scientists have historically had a tendency to work from the information deficit model: if you provide people with the facts, their minds will change to fit your position. But that’s not really how people work. Having accurate information is a necessary condition for making an informed choice, but it is not sufficient. People make decisions not just based on the facts (or more accurately, what they perceive to be the facts), but also based on their values- and values are not always shared between the experts and those asking them questions. The missing ingredient? Trust. But that gets increasingly more difficult as people’s misperceptions become imbricated with and coalesce around a particular identity. Now, any perceived challenge to those misapprehensions you are trying to correct is an assault on deeply held values. But it’s not hopeless. In fact, I was imbued with a profound sense of hope listening to the recent “Why Should I Trust You?” podcast episode between public health leaders and MAHA movement acolytes because, it turns out (to my immense surprise), there are far more points of agreement than disagreement. One comment in particular struck me: the work being done by scientists, work that many people would consider very valuable and important in their daily lives (such as how environmental exposures may contribute to the development of neurodevelopmental disorders) simply is not filtering down to the people who care about it. The epistemic bubbles we live in have hard shells. It will be extremely difficult to make headway in getting to a better tomorrow for everyone until we can break through those shells- and the answer to how to do that isn’t going to come from a study about the science of a particular ingredient. It might, however, come from a study about epistemic bubbles and persuasion.
Antigen and immunogen mean essentially the same thing but strictly speaking, an antigen is anything that your immune system can recognize, whereas an immunogen is an antigen that induces an immune response
This point may seem confusing in light of the recommendation for updated COVID-19 vaccines every year and the concerns about waning antibodies, so I want to clarify this: the immunity elicited by mRNA vaccines themselves is very robust and durable. Memory B cell responses in particular are excellent and CD4 and CD8 T cells against the spike protein remain stable for many months. The antibody response does wane, which is expected with any vaccine as there is always an initial burst of antibodies from plasmablasts and short-lived plasma cells, and then steady-state antibody titers are maintained by long-lived plasma cells as I discussed here. Whether the antibody response wanes more quickly than it does for other types of vaccines is not entirely clear right now (there is some suggestion that this could be the case when looking at RSV vaccines, but with different assays performed by different groups, direct comparisons are difficult; it is also possible that there are differences in mucosal immunity elicited by the different vaccines that are not being captured with current data), but available evidence suggests it doesn’t substantially differ from that of Novavax. The issue here though is that SARS-CoV-2 is a rapidly replicating, mucosal infection and it is constantly undergoing antigenic drift to escape pre-existing antibodies. This means that to durably suppress infections and transmission, you need high levels of (neutralizing) antibodies at the site of infection, which is not realistic and may not be realistic for any vaccine regardless. Protection against the severe disease remain robust even without boosters and even with highly drifted variants because there is enough time for the immune system to mount a recall response to control the infection at this point (note: because of changes to the immune landscape with the emergence of Omicron variants, measured effectiveness values may understate the degree of protection conferred by vaccination).
This has become something of a bugaboo with some people, so I want to explain this a bit more with an analogy. Think about a book. You can read the book cover to cover and get a detailed understanding of its contents. Suppose now, you rip out a random page from the book. You can still read the page fine, but without the context of the rest of the book, it’s much more difficult to infer what the meaning of the page is, let alone what the book is about. Imagine now we shred the book into a bunch of small pieces. It is unrealistic that you could now piece together the meaning of the book from these pieces alone. The reading of a book is similar to how DNA works. Fundamentally, its a sequence with a specific alphabet that gets interpreted into a given message. During the process of vaccine manufacturing, DNA is treated with enzymes called DNases to shred the DNA into a bunch of smaller fragments. That means that our cells can’t really deduce the meaning of that code anymore. You can’t get the DNA from the cultured cells to be interpreted into a coherent message. Plus, should that DNA get inside our cells, it will undergo further shredding to the point of being reduced to single nucleotides (letters; see hyperlinked post for details). In other words: there is no plausible risk from the very small residual quantities of DNA that are present in vaccines.
I have seen credible sources report ~4.4 mg after the first 6 months. Here’s my math: To compute the 3.18 and 1.58 mg I got, I manually went and looked at the childhood vaccination schedule and every vaccine that can be offered via the vaccination schedule by 6 months (I made sure not to use any brand vaccines that are not approved for use for 6 months of age or younger), found the aluminum content of each, multiplied by the number of doses that can be given by 6 months of age, and found upper and lower bounds. From these I got:
Hep B (3 doses):
(DTap/polio/HepB) Pediarix- 0.7 mg Al (from 6 weeks of age, 3 doses) = 2.1 mg Al
(DTap/Hib (PRP-OMPC)/polio/HepB) Vaxelis- 0.319 mg (from 6 weeks of age, 3 doses) = 0.913 mg Al
Engerix-B- 0.25 mg (3 doses from birth) = 0.75 mg Al
DTaP (3 doses):
Infanrix- 0.5 mg (x 3 doses) = 1.5 mg Al
Daptacel- 0.33 mg Al (x 3 doses) = 1 mg Al
Pentacel (DTap/polio/Hib)- 0.33 mg Al = 1 mg Al
Hib (2 doses)-
PedvaxHIB- 0.225 mg = 0.45 mg
ActHIB- 0
Hiberix- 0
Pneumococcal (3 doses)-
Vaxneuvance (PCV15)- 125 mcg x 3 = 0.375 mg
Prevnar 20 (PCV20) - 125 mcg x 3 = 0.375 mg
Figuring out the aluminum burden contributed by hepatitis B vaccines is a bit complex. Engerix-B is the only HBV vaccine approved for the birth dose, which means we get 0.25 mg Al from that. Per the childhood vaccination schedule, HBV vaccine is to be performed within 24 hours of birth (12 if the mother is positive for HBV infection), at 1 to 2 months, and then at 6 months, for a total of 3 doses. However, there are combination vaccines such as Vaxelis and Pediarix which can also be used for HBV vaccination- but they also cover 5 or 4 (respectively) more vaccine-preventable diseases on the schedule which all require either 2 or 3 doses depending on the specific vaccine-preventable disease. Thus, it is not unusual (in the interest of reducing the number of shots kids have to get) to give Pediarix or Vaxelis at 2, 4, and 6 months- which means that kids functionally get an “extra” dose of HBV vaccine and Hib vaccine. This does not violate vaccination schedule guidelines:
Administration of 4 doses is permitted when a combination vaccine containing HepB is used after the birth dose.
If we assume Engerix-B is followed by 3 doses of Vaxelis, we do not need any DTaP or Hib vaccines in addition to this, but we do need a pneumococcal vaccine (polio vaccination is also recommended in this age group but vaccines for polio do not have aluminum unless part of a combination vaccine which does). This would give 0.25 mg + (0.319 mg)(3) + (0.125 mg)(3) = 1.58 mg Al. This is the lowest possible level of exposure that children could have to aluminum and still meet the vaccination requirements by 6 months.
Alternatively, to get the most Al exposure we would need to do Engerix-B for the first dose, followed by 3 doses of Pediarix, with 3 doses of a pneumococcal vaccine, and 2 doses of PedvaxHIB which gives us: 0.25 mg + (0.7 mg)(3) + (0.125 mg)(3) + (0.225 mg)(2) = 3.18 mg Al.
I computed this from the Ksp of AlPO4 by finding the molar concentration of Al3+ and converting it to a mass via the formula weight of the ion.








Edward:
Awesome article, Thanks!