There's a lot of hype around nasal COVID-19 vaccines. How justified is it?
A look at the latest study (from Sun and colleagues) getting attention.
The first-generation of COVID-19 vaccines have done an extraordinary job in stemming the devastation of this pandemic and to suggest otherwise is simply delusion. But they’re not perfect, and we have at this point identified a potentially important gap in the protection they elicit. Fundamentally, SARS-CoV-2 causes infection by being inhaled into our nose, and more serious problems occur as it migrates downward into our lungs. Suppressing SARS-CoV-2 at the early stages requires a robust immune response right at the site of infection, but the current vaccines do not induce responses localized to this site (at least, in people who have never been infected before). On this basis, a number of next-generation vaccine approaches are being considered, and one of the key ones is nasal vaccines. The concept here is easy enough: deliver a vaccine to the site of infection to induce immune memory that lives right at the site of infection (read below to understand what I mean by “lives there”). This, theoretically, should allow for more effective suppression of transmission of the virus. But the practical aspects are more complicated. One recent study examined such a vaccine candidate (NB2155) in healthcare workers in China who had previously received inactivated SARS-CoV-2 vaccines and it has people really excited. Unfortunately, as is often the case, the excitement far outpaces the data. Let’s examine this paper together.
Background
Before digging into this paper, I wanted to spend a minute discussing the thinking in terms of the immune system behind why some believe nasal vaccines might work better. When we have an immune response, a portion of the cells we activate stay behind and persist for a long period of time. Then, if we encounter the same threat again (or one that’s similar enough) these cells can rapidly be recalled to control that threat more quickly. Such cells are known as memory cells. Broadly speaking, when we talk about memory cells we are referring either to memory B cells or memory T cells. B cells give rise to the cells that secrete antibodies. Some of these cells can live for a long time and maintain antibody levels for their lifetime (these are known as long-lived plasma cells). Memory B cells can also rapidly differentiate into antibody-secreting cells (more quickly than B cells that have never seen their target before; these are known as naive B cells). T cells are a bit more complex. T cells can have the role of helpers or killers. Killer T cells can eliminate infected cells to interrupt the spread of viruses. Helper T cells tell the other parts of the immune system what to do, including the B cells.
Our current COVID vaccines do a fantastic job inducing memory B cells and memory T cells, and these will generally work for almost everyone to step in and stop serious illness from setting in if an infection occurs unless any combination of the following occur:
there is a drastic change in the virus
there are substantial comorbidities in place that accelerate the time to serious illness
there is something that interferes with the function of the memory cells (such as immunosuppressive medications)
However, one of the complicating aspects of memory is that it comes in many different flavors. These distinct flavors of memory cells can be understood (in part) in terms of how the memory cells migrate in the body. For this we only care about the tissue-resident memory cells, but to understand these we need a basic idea of how they differ from the other types so please bear with me. Below a summary is shown for memory T cells, for which these flavors are most well-defined, but analogous rules apply to B cells:

Naive T cells (T cells that have not encountered the antigen they respond to yet) reside within the lymphatic system in the lymph nodes (secondary lymphoid organs, SLOs in the image) at a site called the T cell zone (TCZ) and can migrate into the blood.
Central memory T (Tcm) cells reside predominantly in the lymph nodes (SLO) and can move into the blood.
Effector memory T cells (Tem) can go all throughout the body, including into non-lymphoid tissue (NLT)- basically this means anything that is not part of your lymphatic system, like your respiratory tract, your GI tract, etc.
Tissue-resident memory T (Trm) cells are memory cells that persist at the site where they are first activated. If you have a SARS-CoV-2 infection, you will find these primarily in your respiratory tract, for example.
Analogous principles apply to B cells. One of the major goals of an intranasal vaccine is to elicit tissue-resident memory B and T cells right at the site of infection, which current vaccines do not do (unless you have already had COVID-19 or have certain microbiota).
Another issue has to do with antibodies and it’s a bit nuanced. In the blood, the most abundant antibody is IgG. In the upper respiratory tract (and most mucosal tissues) it is IgA- but specifically, it is secretory IgA (sIgA). Even though IgG can get into the mucosal tissues through a number of mechanisms, it is much less abundant than IgA and in that environment, it does not persist as long as IgA (the opposite is true in blood). Furthermore, though you do find IgA in the blood, this IgA cannot get into the mucosal tissues1. IgA has to be made by antibody-secreting cells that live right in the mucosal tissues to get to them.

When we initially get vaccinated, there is a drastic rise in the level of spike-specific IgG and this is enough to protect us from infection by circulating variants for a time- but as its levels decline in the blood, they decline in the upper respiratory tract as well. Because our current COVID-19 vaccines are not administered into the nose, we do not get production of sIgA that could rapidly arrest infection right in our noses (unless we have been infected before or have certain microbiota). Thus the hope is that nasal vaccines could fill the gaps of (1) Trm/Brm/mucosal plasma cells and (2) sIgA against SARS-CoV-2. Basically, we want a picture (below) more like the one on the left than the one on the right:

In slightly more technical terms, the diagram below explains the differences as well (parenteral meaning not taken by mouth, in this case referring to injection):

With that out of the way, we can talk about the findings of the paper.
The Paper
The vaccine is a replication-incompetent adenovirus vector (like Johnson & Johnson/Janssen and Oxford/AZ) that had the BA.1 spike (an old Omicron variant). The first dose of vaccine was scheduled to be given November-December 2022.
The trial itself is small: 128 healthcare workers who had received 2 or 3 doses of inactivated SARS-CoV-2 vaccines and did not have a history of infection were recruited to get the first dose. The average age of everyone here was about 35.
Of these, 45 reported infections before they could get the second dose and 8 were lost to follow up from the study. That left us with 75 people.
The team was able to get data on the immune response from 63 of them, but 43 reported infections on days 15-28. After all of that, just 31 were eligible to get the second dose. By the end of things, 28 had managed to get out with no infections at 3 months.
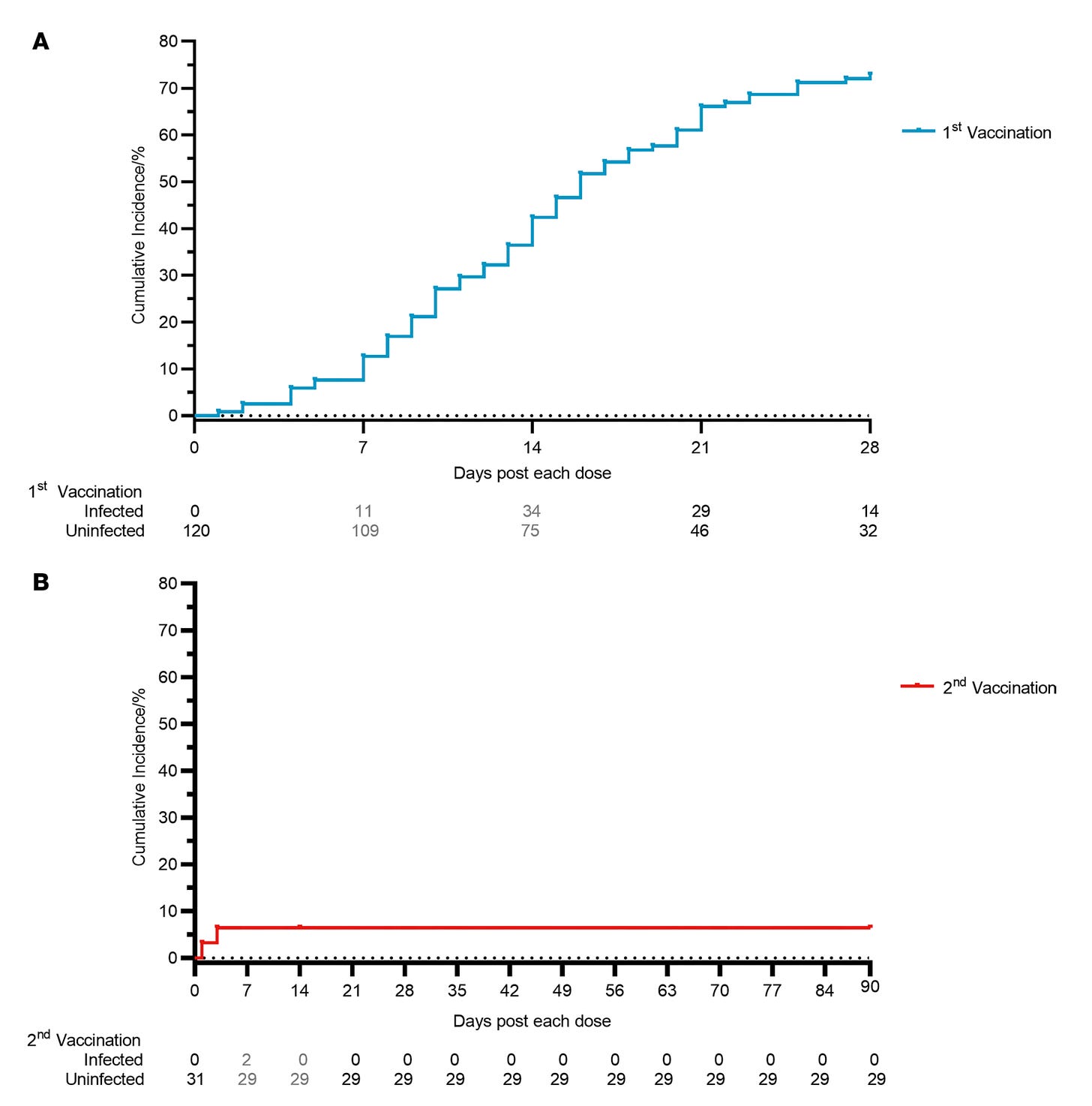
As it was, the trial was definitely too small for an efficacy assessment, but with so many people dropping out before they could start measuring efficacy, there is a very strong survivorship bias component here. The people who dodged infection this long could differ in important ways from those who did not. The trial also didn’t have a placebo group for us to compare incidence with, so I don’t think we can conclude much of anything from that.
Nonetheless, the vaccines were well tolerated:
The major data of note here are the immunogenicity data. In this group of healthcare workers, there was solid induction of antibodies against a bunch of variants. Panel A (below) is the nasal fluid. You can see that at day 42 there is a big jump and at day 118 it is still elevated compared with day 14 but still a lot lower than for day 42. Note that these are just binding antibodies- not all of these will stop infection. Panel B and C are both serum neutralizing antibodies with pseudovirus and authentic isolates respectively. Basically the key point here is that the vaccines mainly induce a response in the nose- not so much inside the body.
The team does note that this response is significantly broader than what has been seen with other intranasal adenovirus vectored vaccines for COVID. They hypothesize that the strains of adenovirus used for these vaccines might not be well adapted to the respiratory tract (not sure how much that matters here though because the vaccines do not replicate in any of these cases). Adenovirus-5 (the one this vaccine is based on) generally does do a very good job with generating immune responses in people compared with other strains though.
There was no assessment of the ability of the vaccine to induce TRMs/BRMs/mucosal plasma cells.
There is also a cumulative incidence curve curve for infections but I honestly don’t know that you can conclude anything from it given the absence of a placebo group and the strong survivorship bias.
So basically: this vaccine induced (some) immune responses that we think would be likely to protect fairly well against infection and this limited group of people tolerated it well. It does seem like 2 doses of the vaccine given 28 days apart however were needed to get this kind of response. I don’t think anything can reasonably be concluded about how well the vaccine actually blocks infections from the data we get here. It’s also worth noting that the variants tested here are broadly speaking, old, relative to what we see today. We don’t know how well this vaccine would induce protection against these current variants from these data. I suspect that it would still need an updated spike protein. We also don’t know how the immune responses would compare with different vaccines (i.e. those primed with mRNA as most of the people reading this have been). It could be the case that the boost would be poorer because the mRNA vaccine-elicited immune responses might eliminate the adenovirus infected cells more quickly and reduce the amount of spike in the nose to mount a response against, for example. Basically: it is maybe promising, but we need way more data before we can say anything with confidence.
Outlook
I am not convinced that nasal vaccines will be a game-changer in terms of the pandemic, to be frank (at least by virtue of the fact that they are nasal vaccines alone). The basic reason is simple: nearly everyone in the US already has hybrid immunity, meaning they have relatively high levels of these Trms/Brms/mucosal plasma cells and sIgA- but reinfections still happen and the public health burden is significant. The virus’s evolution remains a problem: it evolves to escape prior immunity. It is not unreasonable that nasal vaccines could enhance the duration for which people are protected from infection, but it does not seem realistic to me that it heralds an end to the need for boosters if the goal is durable suppression of infections in general. Given that hybrid immunity is already pervasive though, and we see that the elicited immune response from current vaccines does localize to the upper respiratory tract as well, how much of a benefit do nasal vaccines really offer over our existing methods? The current data we have, be it this study from Sun and colleagues or elsewhere in the literature, do not yet address this question. Still, the work is not without promise and it is worth pursuing further.
This is because IgA in the serum is mainly monomeric. To get to the mucosal tissues, it needs an additional protein called the J-chain (J stands for “joining”), which is found with IgA that is dimeric. This allows it to be recognized by a protein called the polymeric immunoglobulin receptor (pIgR) and enables it to be imported to the mucosae.





Excellent run down thank you.
The 30 or so subjects that made it through the bottleneck of Covid surging in China to receive the second dose could absolutely be “unique” in risk avoidance behaviors or immunity.
I am optimistic that even with hybrid immunity in the nasal mucosa of most people, giving a short term boost there will hopefully reduce infections (zero of those 30 is still impressive in the context of a surge that caught 85% of the Chinese population).
Also, hopefully priming the defenses at the front door will lead to lower viral loads and faster clearance of coronaviruses, both associated with less collateral damage and long covid.
I’m still optimistic these will be net positives if not game changers, and such platforms will need to be nimble to keep up with mutations in spike. Hope someone is targeting other conserved antigens/pancoronavirus epitopes?
Ps - the grandeur, science, and terror of the immune system are incredible, thanks for the reminder.