Testing out of isolation when you have COVID-19 has become really complicated
People want to *know* they're no longer contagious before resuming normal activities- but there are certain scenarios that make it very difficult to conclude that.
Some time ago, the US CDC sparked outrage from some when it updated isolation guidelines back in 2022 (current guidelines here and FAQs here): 5 days of isolation, followed by 5 days of masking if symptoms have improved and no fever is present (a major aspect to the outrage seemed to disregard that latter recommendation). Some contended that rather than make guesses about when a person was no longer infectious, rapid antigen tests could be practically implemented as a guide- people could leave isolation once they had serially negative rapid antigen tests1. But there are problems with that idea. Simply put, there is no practical test currently that can guarantee you are no longer infectious if you get COVID-19 (or any other respiratory virus, for that matter), and it seems from the available data that, implementing them as a basis for decision-making about isolation carries meaningful risk of both people being isolated for longer than they need to be and people leaving isolation while they are still infectious. Rapid antigen tests are most likely to give accurate information about infectiousness when a person has symptoms, especially a fever, and are appropriate to use after a known exposure. For the average immunocompetent person, the CDC’s current guidelines (basically: wait until you feel better, then wear a well-fitted mask for 5 days and take additional measures to improve indoor air quality) are broadly reasonable. Details are provided below.
The following is best understood if readers have a reasonable familiarity with the nature of medical testing; for those who may not, please see this foonote2.
Broadly speaking, there are two3 major types of tests for detecting SARS-CoV-2. The first are the nucleic acid amplification tests (NAATs), sometimes known as “molecular tests,” but I don’t think that term provides useful information, or “transcription-mediated amplification tests” (TMAs). These include PCR (technically RT-PCR because SARS-CoV-2 is an RNA virus, and the quantitative version, RT-qPCR) and isothermal amplification tests; while they are highly sensitive, they can remain positive for well past the point of infection, which means they are not necessarily helpful for answering the question of whether or not a person is infectious. However, if a person is very high risk for COVID-19, for example, and thus the priority is to initiate antivirals as soon as possible, PCR may be the best test for that question because it is more likely to give a positive result early on in the illness than other tests. The alternative to these are the rapid antigen tests.
Before getting into the data, I should state explicitly: if the choice is between using any kind of test vs. no testing whatsoever, there is an obvious public health benefit to testing in that you will be putting infectious people in isolation, disrupting transmission chains. Using multiple tests throughout the course of an infection increases the sensitivity of the test i.e., it is more likely that at least one of the tests will be positive, reflecting SARS-CoV-2 infection. This is important to bear in mind because if you test too early, antigen levels might not be high enough to give a positive band on the antigen test, producing a false negative.
The majority of rapid antigen tests work by detecting the presence of SARS-CoV-2’s nucleocapsid (N) protein.

In general, the N protein has remained very stable throughout the evolution of SARS-CoV-2, so we don’t really have to worry about the virus evolving to escape detection on rapid antigen tests (although continued monitoring is prudent). While there is variation by specific test type, broadly speaking the tests have an antibody against the N protein, which clumps together when N is present and produces a dark band (there’s variation across tests regarding the biochemical details of how the band forms but this is the basic idea4). It is true that one of the things that makes rapid antigen tests so valuable is that positive results correlate very well with virus culture (i.e., plaque assay or similar). The presence of virus on plaque assay is more or less the gold standard for determining whether or not a sample is infectious. Unfortunately, it is completely unrealistic to implement plaque assays at the public health scale (they literally take days and are very resource intensive in comparison to PCR and rapid antigen tests), meaning that they not actionable as a basis for decision-making. This suggests that rapid antigen tests would be a reasonable proxy then for infectious virus, given their high correlation with the results of virus culture. This does seem to be true early on in the infection, but it gets muddier later.
Intuitively, given the previously noted correlations between antigen test results and virus culture, one would think that a positive rapid antigen test indicates continued infectiousness, and so, if we act in the vacuum of best practices for infection control, a positive result translates to “keep isolating,” and a negative result implies “leave isolation.” False positives and false negatives (as defined by antigen test agreement with virus culture) are both very troubling here. False positives would keep people in isolation longer than they need to be- which is fine if you have the financial means and latitude in your responsibilities, but that is likely rarer than advocates of this approach appreciate. False negatives mean sending people out into the world while they are still infectious, potentially leading to continued propagation of transmission chains. Unfortunately, we have significant data gaps regarding how to interpret rapid antigen tests that remain positive for longer than we expect, and even negative antigen tests can be untrustworthy under some conditions, as I explain below. Note: all of the following assumes immunocompetent study participants in the studies unless otherwise stated.
Most data examining antigen tests serially and comparing them to virus culture are from 2021 and 2022, largely before the Omicron variant. In one study using serial sampling, antigen tests were compared with RT-qPCR. The study also assumed that Ct values greater than 325 on RT-PCR would produce negative viral cultures based on prior data, which is an understandable shortcut given the constraints of culturing SARS-CoV-2 (i.e., BSL-3 labs), but I think warrants caution in interpretation.
Sensitivity (based on RT-PCR as the reference) varied some by variant (although confidence intervals are relatively wide):
This is likely not an effect of the variants mutating to escape detection on rapid antigen tests, but rather the changing dynamics of immunity in the population as the pandemic progressed and possibly (though less likely for this set of variants) differences in the behavior of variants in terms of where and how quickly they replicate. There are several studies like this, but these studies are based a lot on pre-Omicron variants. Because of the period of time in which these studies were conducted, most of the people in them were not previously infected, whereas today nearly everyone has been infected at least once, and probably with some flavor of Omicron.
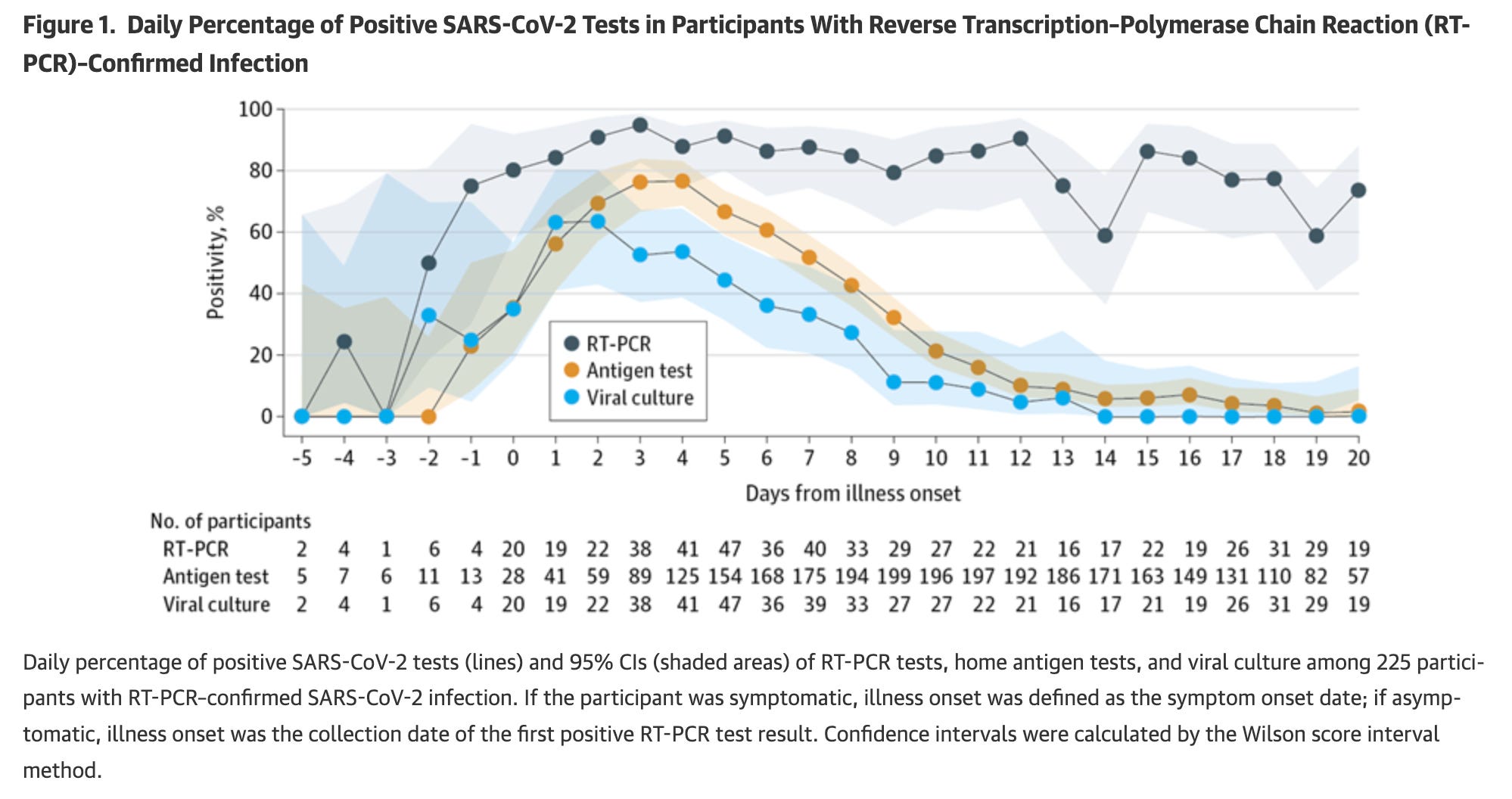
When Omicron showed up, interpretation started to get more confusing. One study of healthy college students who were vaccinated found that at days 4-6 from the first positive result, a positive test result reflected a positive virus culture 50% of the time, which seemingly led to some experts to suggest that antigen tests are not reliable past day 5 if they are positive. This result, however, is based on just 8 positive rapid antigen tests, and includes both Delta and Omicron variants. Intuitively, the longer you go past this point, the less likely a positive result is to be a true positive. Furthermore, virus culture remained positive on days 6-10 in 17% of individuals, and in some went as far as 15 days from the time of diagnosis, which authors note supported the guidance from CDC to mask on these days even if you leave isolation:
Then, things got even more confusing. In a study through the ACME-POCT network, from April 2022 - April 2023, participants underwent serial PCR testing to examine trends in viral load (this is necessarily fraught with PCR because it cannot distinguish between subgenomic and genomic RNA):
The key finding here was that Ct values tended to dip later in the course of illness (remember- higher Ct values indicate less of the target sequence was initially present, suggesting, though not proving, less virus) relative to prior variants, perhaps indicating the effect of immunity in launching an earlier response to the virus. Nonetheless, this was in stark contrast to earlier pandemic data which suggested that viral load tended to be highest around the time of symptom onset. Importantly, antigen concentration did seem to drop significantly at around day 5 since symptoms began in this study, but strangely bounced up again on day 6 before dropping again on day 7. The interpretation here with respect to infectiousness is thus not at all simple, and unfortunately, there was no serial virus culture attempt to assess the duration of infectiousness.
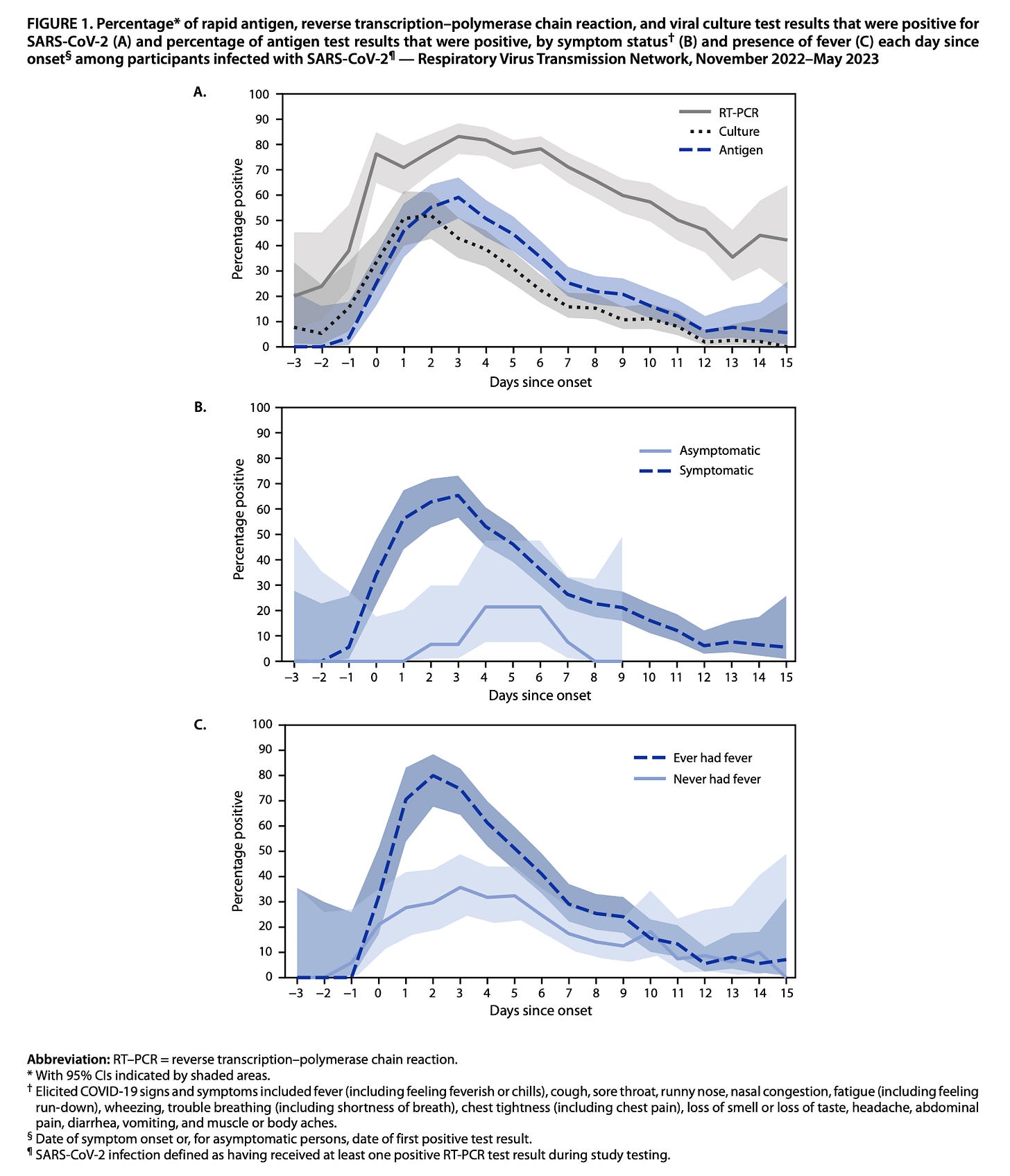
Another key study came from the CDC’s respiratory virus network, which looked at performance of serial rapid antigen tests compared with PCR and virus culture (actual virus culture with Vero E6 cells expressing ACE2) from November 2022–May 2023. The critical figure is this one:
While the figure does not show the degree of concordance between the testing types (i.e., as a worst-case scenario it could be that none of the positive results overlap for antigen tests and virus culture when the rates of either/both are below 50%), it is noteworthy that even though this is firmly in the Omicron era of variants and there was at this point extensive population immunity, there is a very similar proportion of antigen tests that are positive when virus culture is positive. Importantly, test sensitivity (i.e. the likelihood of a positive result occurring if the virus culture is positive) was highest if fever was present in particular, and if symptoms were present. With fewer symptoms, the sensitivity of the test dropped some, meaning that people who had positive virus cultures had negative test results (94% sensitive if fever was present, 45% sensitive if no symptoms were present). That’s potentially concerning because suggested that people who were infectious could be going out into the world inappropriately reassured by negative rapid antigen tests (again, underscoring the importance of continued masking in public past the period of isolation).
This was also seen in a cohort study of rapid antigen tests in Germany which were compared to RT-PCR: the presence of symptoms significantly improves test sensitivity.
Still, it is noteworthy that performance was similar regardless of specific antigen test used here, which is reassuring. The fact that the gold-standard being compared to here is RT-PCR, however, is not as helpful for questions of infectiousness.
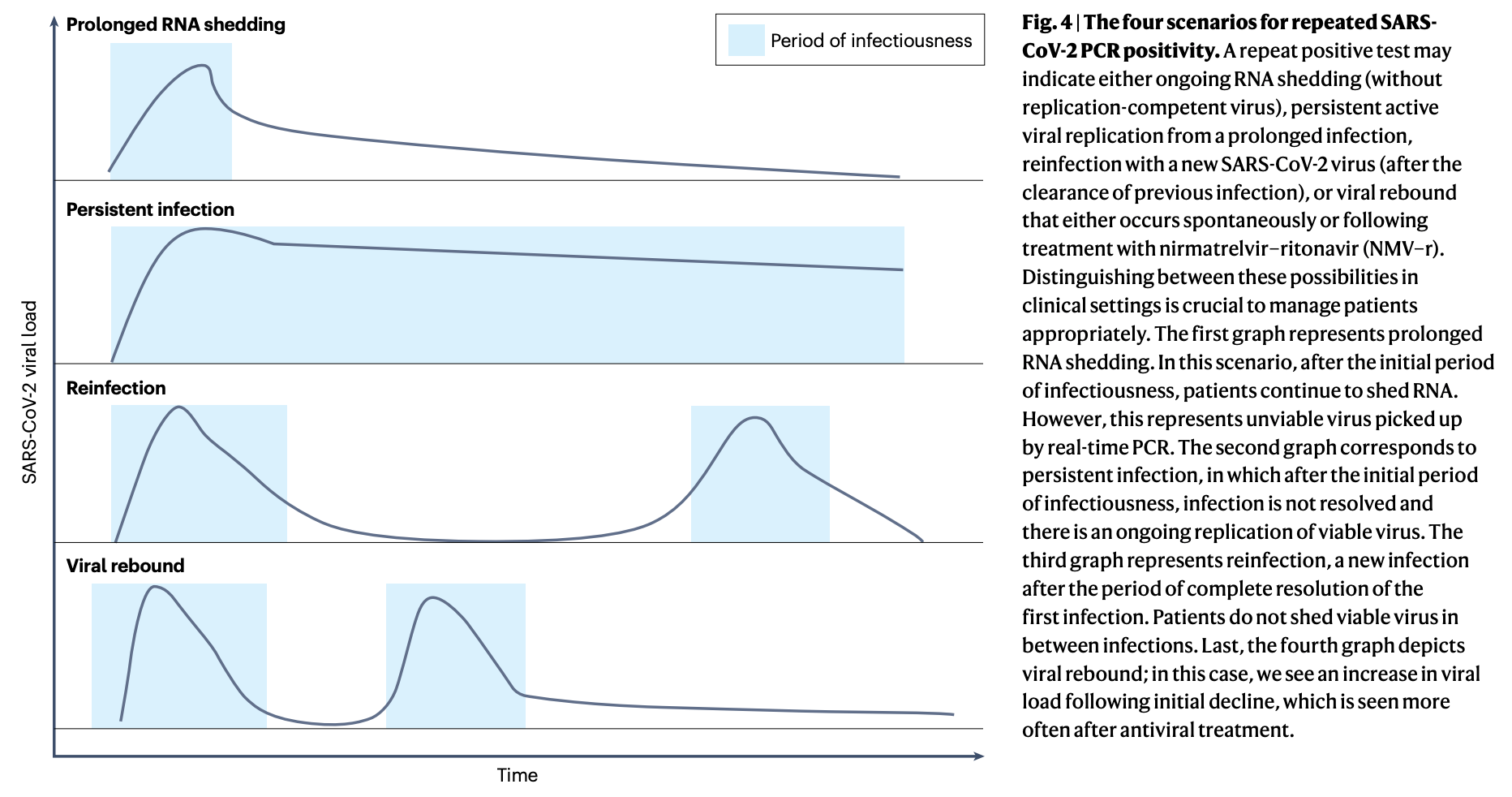
In a small but very much real subset of people (13 people out of 11,297 in the study; details on the cohort are described here), after having COVID-19, rapid antigen tests simply stay positive for significantly longer than you expect them to (in one case as late as 53 days). While it is more common in women and those with autoimmune diseases, some people appear to, in the course of their illness, make an antibody that reacts with substances in the test (such as the antibodies on the test itself, as may occur with rheumatoid factor), inducing a false positive result.

It should be noted however, that rheumatoid factor shows up transiently in many people during infections, so a single positive test result should not be presumed to be indicative of rheumatoid arthritis or other autoimmune diseases. The authors who report this finding suggest trying a different brand of antigen test. Personally, I am not sure about that recommendation as, if the test still uses IgG antibodies against the antigen (usually nucleocapsid) to detect it (as I believe all the rapid antigen tests do, but I may be mistaken), rheumatoid factor would likely give a positive result with any of them. Alternatively, PCR could be used here, as a negative result on PCR when an antigen test is positive strongly suggests that there is not infectious virus present. The downside here of course is we know PCR can be positive for up to 3 months even in immunocompetent people, so you very well could get a positive PCR entirely by coincidence. Another possibility is to test for antibodies against nucleocapsid directly as proof of infection, but this has two issues. The first is that, at this point, nearly everyone already has antibodies against nucleocapsid because nearly everyone has had at least one infection (even if they didn’t necessarily become ill). However, we also know that some people can have infections that are so effective in suppressing virus that they do not generate enough nucleocapsid antigen in the course of their infection to make a detectable level of nucleocapsid antibody (Moderna used nucleocapsid antibodies to examine how well its vaccine blocked infection in its phase 3 trial).
It is also worth noting that on occasion, virus load has been observed to rebound. Even though Paxlovid is best known for this, it does occur in the absence of treatments as well (for example, the placebo group of the Paxlovid trials also experienced some rebound, as determined by RNA copy numbers). Rebound has been associated with a return to culture positivity, and may result in a positive rapid antigen test.
In short, interpretation of a prolonged positive antigen test, while it applies to a rare subset of the population, can be very complex.
An alternative approach to isolation decisions could be to consider how long people are likely to produce positive virus cultures in general, regardless of testing. Here there is a good deal of heterogeneity in the data. It seems that the average time to a negative culture based on pooled analysis of studies is 5.16 days for the Omicron variant, counting from the onset of symptoms; assuming that the distribution of infectivity follows a normal distribution, this would mean that roughly half of people have infectious virus on day 5 and half do not. On day 7 about 1 in 5 people still had positive virus cultures. A study of infected healthcare workers however noted that a critical modifying factor in the duration of infectiousness was whether or not the infection in question was the first one or a recurrent one; first (primary) infections tended to be infectious for substantially longer than recurrent ones:
35% on day 5 for recurrent vs. 79% for primary
5% on day 7 for recurrent vs. 55% for primary
0% on day 10 for recurrent vs. 22% of primary
Consistent with this, one study has shown that the development of mucosal secretory IgA was the strongest predictor of the development of negative virus culture for Omicron BA.1 and the latency to a mucosal IgA response was a key predictor. As of this point, the only way to elicit mucosal secretory IgA has been infection, and indeed, prior infection predicted the time to the development of an IgA response. At this point, most people in the US have had at least one case of COVID-19, meaning that it is likely that the parameters for the secondary infections are the more germane ones. Based on the healthcare worker study, it seems that a large majority of people are no longer infectious by day 7 of symptoms, although a small number can be. Nonetheless, to the best of my knowledge whether this is still true for current variants seems unclear to me.
I do feel the need to point out that some argued that these isolation guidelines showed that the CDC was capitulating to economic forces, rather than doing what was best for infection control and therefore public health. Whether or not these guidelines were informed by economic considerations like lost productivity from absenteeism from work (which I don’t think anyone who was not involved in drafting the guidelines can really argue definitively), it is important not to overlook the basic fact that socioeconomic status has profound consequences for health. It is very much the case that many people in the US live paycheck-to-paycheck and do not have generous paid time off policies. Missing too much work can mean grave financial jeopardy, particularly if it results in loss of employment, that in turn means worse health outcomes. For example, many receive health insurance through their jobs and require it to be able to get care and fill their prescriptions to maintain their health. Furthermore, much essential work is, simply put, essential. Extensive absenteeism can threaten access to goods and services that are critical for our daily life. While the threat of a pandemic virus is a critical consideration, approaching public health from the totally sterile lens of infection control as the only priority does disregard other very real harms to the wellbeing of society. It is for this reason that it is critical to get legislation that protects paid time off for illness. While we should vociferously argue for this kind of change, SARS-CoV-2 was not going to wait for us to get our house in order before continuing to explode in transmission chains, so something had to be done to balance these competing risks.
When it comes to tests in medicine, every test has a defined sensitivity and specificity (note that tests here refer to all forms of medical testing, not just things like labs- this can include things like the presence or absence of a particular sign or symptom, for instance). The sensitivity is the likelihood of the test giving a positive result when the thing you are testing for is present; this gives insight into the false negative rate- higher sensitivity tests have lower false negative rates. The specificity is the likelihood of a test giving you a negative result when the thing you are testing for is not present; this gives insight into the false positive rate- higher specificity tests have lower false positive rates. These measures are always based on comparison to some gold-standard test. No test exists in which both of these will be 100%, but some tests get very close.
It’s important to remember that when we talk about test results, we are often reducing continuous variables (e.g., the level of hemoglobin in the blood, which can span essentially any value, in theory) to binary ones (positive or negative) and this is based on a threshold value (there are tests that give you “maybe” results too, but considering these makes the math much more complicated). Suppose that the higher the value on a test, the more likely the condition is present. If we lower the threshold for a positive result, the test will naturally capture more positive results, making it less likely that we will miss true positive results even if they are less extreme. In more formal terms, lowering the threshold increases the sensitivity of the test: it becomes better able to detect true positive results (i.e. that the condition is present) even if they are less extreme. However, this also means the test will generate more positive results in general, including false positives. Thus, it lowers the specificity. This is broadly true: changes that increase the sensitivity will tend to decrease the specificity and vice versa. The threshold for a test matters a lot because test results decide treatment. See the example in the figure below.

One very common misconception is that if a test is e.g. 99% sensitive, then a positive result will be a true positive 99% of the time, and by extension, a test that is 99% specific will give a true negative 99% of the time. People who argue this are failing to get at the subtle but important distinction between the chance of a positive result given the disease is present and the chance of the disease being present given a positive result. To illustrate the difference, consider the example below:
Suppose we have a condition that affects 1% of the population and we test everyone in the population with a test that is 99% sensitive and 99% specific (and assume that these test characteristics are based on a reference population that is similar to the general population we are about to test it on).
To make the numbers simple, let’s say we have 10,000 people in the population (you can use any numbers but this just makes the math easier) and our test performs exactly as advertised.
That means that 100 of them have the disease.
Of these people who have the disease, 99 will get a positive result, and 1 will get a negative result.
By extension, we have 9900 people who do not have the disease.
Of these, 9801 will get a negative result and 99 will get a positive result.
So, given that you got a positive result on the test, the chance that you have the disease depends on how many people with the disease test positive out of the total number of people tested:
(99 true positives)/(99+99 total positives) = 50%.
That’s right: half the positive results from this amazing tests are false positives. "
This value is known as the positive predictive value (PPV).Using the same logic, the chance that a negative result is a true negative is: (9801 true negatives)/(9801+1 total negatives) = 99.98%.
This value is known as the negative predictive value (NPV).
This kind of work is often summarized in tables:
The counterintuitive part here is many people assume that the probability of a positive result given that the disease is present is the same thing as the disease being present given a positive result- but as this example shows, it clearly isn’t. Broadly speaking, independent of test characteristics (sensitivity, specificity), the rarer a condition is, the more likely a negative result is to be a true negative, and by extension, the more common the condition, the more likely a positive result is to be a true positive, or put another way: things that are common push PPV up (and NPV down), and things that are rare push NPV up (and PPV down).
Key insights from this include:
Even excellent test characteristics (high sensitivity and high specificity) can give you incorrect results a huge proportion of the time depending on the incidence of the condition you’re looking for.
If you do a screening test, you need to carefully consider the incidence of the condition you are examining and the risks to false positives and false negatives.
Simply ordering every single medical test in existence means generating a ton of false positive results that need to be interrogated, potentially involving risky procedures that would not otherwise occur had no test been done (not to mention the insane cost).
More subtly, this offers us some clues about how to get more information out of tests. For example, a positive result on a low sensitivity test is likely a true positive because it requires relatively extreme deviation from the normal range to be flagged as positive; this suggests that if you have a low sensitivity test with a negative result and then a positive result, the positive result may be more trustworthy (but, as always, tests need to be interpreted in the context of the entire clinical picture).
In addition to all of these factors, it is critical to bear in mind precisely what is being tested. For example, during the pandemic, some argued that PCR tests for COVID-19 were bad because they were producing many false positives- but this isn’t really correct.
The basic idea behind PCR is that you have a target DNA sequence (or an RNA sequence which you make a DNA copy of in the case of RT-PCR, as is used for detecting RNA viruses like SARS-CoV-2) which you flank with primers that are complementary to that target sequence. These primers are designed to bind only to that sequence and no other sequences, and are extensively tested to ensure this is the case. You then iteratively replicate the region flanked by the primers until you reach some threshold signal. A false positive with PCR would mean that a threshold signal was reached even though the target sequence was not present in the sample. Barring issues like mishandling of the samples, designing primers that bind nonspecifically, or some kind of problem with the equipment or reagents, it is essentially impossible for PCR to hallucinate the presence of a DNA sequence that is not there. Put plainly: PCR cannot create a “casedemic.” The issue is people wanted to use PCR to figure out whether or not a person was infected at that moment or infectious in general, and this is a question that, on its own, PCR cannot answer. To answer that, you need to consider additional information. For example, a variant of PCR known as real-time PCR (qPCR, because RT-PCR is reverse transcriptase PCR) can report how many replication cycles it takes to get to the threshold signal. If it takes fewer cycles to reach that signal (expressed as a cycle threshold value, Ct or Cq depending on the reference), it indicates that there was more of the target sequence initially present. Thus, a very low Ct value suggests a lot of the target sequence is present, which is not likely if the infection were not recent. At the same time, higher Ct values on RT-PCR do not rule out that a person is infectious (although they are likely less infectious than those with lower Ct values).
I suppose technically antibody tests (aka serologic testing or simply serology) are another method of detecting SARS-CoV-2 depending on the type; IgM antibodies show up at a median of about day 6 post infection; IgG and IgA will appear later- but this gives away the issue. You have to be relatively late into an infection to even have a chance of having a positive antibody result. In addition, antibodies against antigens other than spike are also widespread at this point, so, at this point, a positive antibody result should be presume to indicate a prior, rather than current, encounter with SARS-CoV-2 or vaccination (if the antigen the antibody recognizes is spike).
It is also important to follow the manufacturer instructions regarding the tests because this can result in false positives or false negatives. For example, there was some furor some time ago when students discovered that they could induce positive results on antigen tests by using soda. This is based on the fact that the tests are not designed for use with such acidic specimens, and these specimens may cause the antibodies in the test band to clump together. Misusing a test to engineer a result does not mean that the test in question is bad or flawed. In the same vein, tests not designed for saliva (or other fluid) samples can give erroneous results when using these samples.
A variant of PCR known as real-time PCR (qPCR), or in this case RT-qPCR (because the starting genetic material of interest is RNA, so it must first be converted to a DNA template by reverse transcriptase) can give more information than just a positive or negative result. PCR involves repeated cycles of heating and cooling the sample, during which the amount of your original sample will double with each cycle. The amount of sample you have is tracked using a probe, which produces a signal in proportion to the amount of target sequence present. At some number of cycles, it reaches a threshold value which is known as a Ct value. The fewer cycles it takes to reach the Ct value, the more of the target sequence was originally present. Most PCR instruments will perform about 40 cycles on a sample, and so if the Ct value is very high, it suggests very low levels of the target sequence in the sample, and in the context of COVID-19, implies a person is likely not contagious at that moment because the levels of virus are too low. However, IDSA advises against using Ct values as a guide for infectiousness. The major issue is that Ct values can vary across instruments and depending on the specific target sequence (the abundance of different transcripts from coronaviruses within a given cell varies; the gene for N tends to be the most abundant because of the nature of coronavirus replication which means it is expected to produce lower Ct values than other sequences), which means that a precise threshold for virus culture being positive or negative is not realistically implementable at the public health level. Nonetheless, as described in the main text, Ct values of 32 or higher are generally not indicative of infectious levels of virus, although, again, this depends on the PCR instrument and target sequence.










Ed- so difficult to negotiate the public and these issues. The even once receptive individuals have forgotten Covid19 and it makes it a careful tap dance encouraging testing and then compliance w at a minimum common sense.
I am convinced that we are seeing so many teens testing negative for Covid right now w symptoms, but as we cannot test in office outpatient for rhino/entero, adeno, mycoplasma, we don’t know. Of course the overlap is large and I am left uncertain. Some we can send PCR, which do return within 24-36h now, but many refuse; and the cost is still high.
I appreciate your work.
We have a staff right now, shockingly her first time after being a “Novid” for so long, with fulminant Covid, and trying to figure out how to bring her back in is a struggle- CDC? Too liberal, 10 days? Feels restrictive, some testing? 2 rapid 48 hrs apart, at least 48h after fever, resolving symptoms? It’s hard. We’ll have to play it day by day. I suspect she may test positive rapid Ag for a bit, so likely will land closer to 10 days…
Dr N - Your remarks on test characteristics and disease prevalence were spot on. It is a lesson we clinicians must learn over and over . Thanks .