No, a paper didn't prove the absence of long-lived immunity from SARS-CoV-2 vaccines
A peek into the messy, messy world of long-lived plasma cell (LLPC) science.
A paper was just recently published in Nature Medicine entitled “SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination.” To explain what this means: plasma cells are cells that make antibodies, and the long-lived ones are responsible for ensuring the persistence of your antibodies even in the absence of exposure to the thing the antibodies target. Their absence would suggest that the mRNA vaccines are fundamentally unable to induce antibody responses that last for long periods. On its face, this sounds really bad. Fortunately, the reality is probably not nearly as pessimistic as this paper suggests, but that won’t stop people from misusing the data in this paper. Below, I explain precisely why this paper’s data (and data from other papers on this question) do not support its very strong claims.
To understand this paper we need to understand a bit about how these studies are done. Immunologists use a technique called flow cytometry to identify different types of cells. The details can be really complex but it basically boils down to this: different cell types have different markers on their surface1 (if you can understand this, congratulations, you are now capable of understanding some very complex immunology- not kidding). This technique allows us to define cell types by the markers on their surface2, and then a related technique called FACS (fluorescence-activated cell sorting) can be used to pick out cells that have specific markers on their surface from our samples so that further experiments can be done with them. Most of the time these markers have a name like “CD” and then a number (many of these have common names as well- for example, CD71 is a transferrin receptor (TfR) which helps get iron into cells). When you do this you basically get grids where you call some level of a surface marker as “positive” (+) and levels below that are “negative” (-) and then you iterate until you have a complete set of markers that defines a cell type. In some contexts, instead of “+” or “-” you might see “hi,” “lo,” “bright,” and “dim” used but these all basically mean the same thing. I go through a representative flow plot from the paper below, but honestly the only part you really need to understand about this is literally: different cell types have different markers on their surface.

The cell type we care about here are long-lived plasma cells (LLPCs). Plasma cells are antibody-secreting cells (ASCs) that do not divide and essentially abandon all functions other than antibody production, but they vary a lot in how long they live. Most of your ASCs are not long-lived, and in fact a majority of the ones you induce in an immune response die very quickly, which is why initially there is a burst of antibody followed by a contraction to a stable level. That level is determined by the amount of LLPCs you have- more LLPCs means a higher level of antibodies. When it comes to dealing with infectious diseases, we generally want long-lived plasma cells that can survive for many years, if not our entire lifetimes, so that we always have protective antibodies (maybe- as described below, there are instances where this is less useful, and SARS-CoV-2 is one of them)3. These LLPCs are classically found in the bone marrow, but they can also live in our mucosal tissues. Most of the plasma cells in the bone marrow are not long-lived. This is where flow cytometry comes in: we can separate the plasma cells in the bone marrow by markers on their surface to define different populations, and hopefully there ends up being a set of markers that indicates an LLPC.
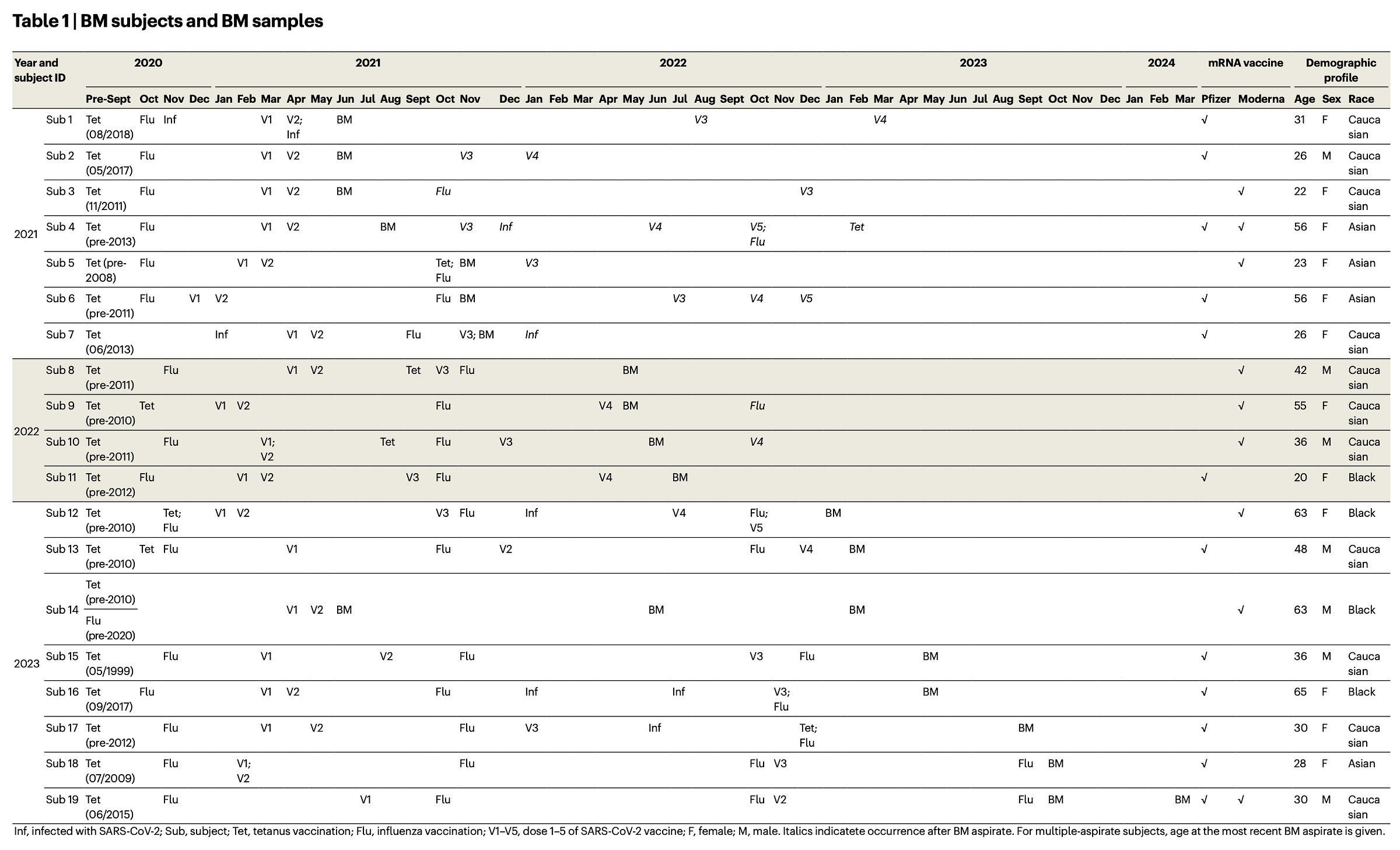
This study had volunteers undergo a procedure where their bone marrow was sampled after SARS-CoV-2 vaccination (and in some cases after infection as well) and looked for LLPCs using flow cytometry according to the schedule below:
Here’s the big problem: there is no established set of markers that clearly defines LLPCs in humans. There is work that suggests that plasma cells with particular markers are more likely to be LLPCs, but at this time, it simply is not possible to do flow cytometry on plasma cells and say “only these bone marrow plasma cells are LLPCs.” The team involved in this work argues that LLPCs reside entirely in the cells from what they call Population D (CD19−CD38hiCD138+), which lack a protein on their surface called CD19. The problem with this approach is that in humans, LLPCs have been identified that have high levels of CD19. In fact, some work has claimed that (in humans) LLPCs can be found in bone marrow plasma cells that have essentially any combination of plasma cell markers. So, while it’s true that this work did not identify population D cells elicited by either SARS-CoV-2 vaccination with mRNA vaccines or infection, it is a huge jump from that claim to the claim that neither vaccines nor infections establish LLPCs, and it is one that other literature suggests isn’t true. For instance, as one plasma cell expert and reviewer of the preprint of this paper put it:
The conclusion that [the plasma cells] are not [sic] long-lived compartment is based solely on CD19 expression may be an over-interpretation of the data. For one thing, if you have ASCs that have been present for 2 years or longer, by definition, they are long-lived.
Other reviewers also pointed out that there was a lot of variation in how the bone marrow samples were collected in terms of timing, which makes it hard to make conclusions. In addition, this team did find many plasma cells that belonged to population B (CD19+CD38hiCD138+). You will notice that the only marker that differs here is CD19, which, again, very well may be present on human LLPCs given other research into the question. The team from this paper even notes that these population B cells might be precursors to LLPCs and might differentiate further into them given more time. In short, claims that this paper shows there is no long-lived antibody response from mRNA vaccines are not justified. In addition, other literature argues against this. For example, one paper found bone marrow plasma cells in participants 17 months after vaccination. Another study tracked antibody titers longitudinally and found, exactly like the textbooks would describe, a period close to vaccination where the titers peak and rapidly drop, and then are maintained at a stable level for many months, consistent with the establishment of LLPCs.
Having said that, we could take a pessimistic view and assume that the paper is totally right (although I don’t think given the totality of the evidence we should). The next question becomes: does this matter? I have written at length before about the value and importance about LLPCs for protection, but there is a key area where they falter. LLPCs are stuck making whatever antibody they have committed to- but suppose now that the virus mutates and that antibody no longer binds. Influenza, for example, in this study, has a very high level of Population D plasma cells from the study participants. Despite that, we are reinfected by flu (on average) once every 5 years, with some people experiencing reinfections far more frequently. Influenza still has a huge public health burden which we help to limit by getting vaccinated with updated vaccines every season. SARS-CoV-2 is not influenza, but, like influenza, it is a rapidly mutating respiratory virus that evolves in a manner that allows it to escape pre-existing antibodies and it will cause reinfections throughout our lifetime in most people. How useful are LLPCs to me if they can no longer recognize the current spike proteins that occur? They’re not. In other words, even if this study is absolutely right about everything (and to be clear, there is very good cause to doubt that it is), it isn’t nearly the bombshell that some people think it is.
The study does get at some important knowledge gaps in the field of plasma cells. For example, it floats the idea that there might be something about the spike protein itself that makes it hard to elicit LLPCs given that much of the study’s cohort was also infected (you would have to accept that the spike protein is indeed bad at this first, though). In particular, they point to the spacing of spike on viral particles not being the proper distance to maximize LLPC production. The counterpoint to this however is: tetanus. The tetanus toxoid vaccine elicited tons of population D cells even though it has none of the properties that have been pointed to as promoting the production of LLPCs. That implies that there is much we still don’t understand about what it takes to get LLPCs. That said, there is one thing about the spike protein that’s a bit weird immunologically. It has been observed (in mice) that when the spike protein enters the lymph nodes so that an immune response can be organized against it, it seems to be taken up mainly by medullary macrophages. Normally, we associate strong immune responses with uptake by a different type of macrophage called subcapsular sinus macrophages. Whether or not this translates to a meaningful difference in the immune response to spike protein is not currently known.
Lastly, this should not be taken to mean that we should not work to get better immune responses from our vaccines or against SARS-CoV-2. In particular, studying the immune response to mRNA vaccines in humans is difficult because it differs in how it behaves long-term from that of mice. With mice, even one dose of mRNA vaccine gives essentially permanent antibody titers at a very high level- this is not the case for humans and unfortunately we do not yet know why. It is also possible in principle that mRNA vaccines may not be as good at inducing LLPCs as other types of vaccines. This is potentially suggested by the data on RSV vaccines for older adults, wherein the mRNA vaccines induce antibody responses that seem to fade more quickly than those induced by either protein vaccine. Figuring out how to make mRNA vaccines work as well as possible remains a critical area of research that should not be neglected. Nonetheless, this study doesn’t suggest that they work poorly (it’s not the right design for that anyway), and in fact, it’s not even clear that this study identifies a true gap in the immune response they elicit.
You can also use flow cytometry to look at markers that are inside the cells but it’s more complex and not always feasible. One approach here is to link the marker to a fluorescent protein, but this means you have to go and manipulate the genome of the organism, and for humans that isn’t feasible. Alternatively, you can fix and permeabilize cells to let antibodies penetrate into them to visualize markers inside of cells, but this kills the cells in question, which generally means you can’t do further experiments to learn more about those cells. In particular, one key technique is known as intracellular cytokine staining. Cytokines are proteins that cells secrete, but the trouble is even though you can easily identify a cytokine once it’s been secreted, you have no way of knowing which cell it originated from. To get around this, you can isolate a particular cell type, stimulate it, and treat it with a substance that blocks secretion. After this, you can fix and permeabilize it and then measure the levels of cytokines inside the cell.
To give an example of how this works: T cells all have a protein on their surface called CD3. Once you have identified the cells that have CD3 you can get more granular. For example, mature T cells have either CD4 or CD8 on their surface which defines whether or not they are helper or killer T cells respectively. As this table shows, flow cytometry allows for very granular characterization of different cell types:
It’s important to emphasize that a lack of LLPCs does not automatically mean a lack of protection against all outcomes of an infectious disease beyond the short-term. The immune system is not just antibodies- memory B and T cells are another critical component for example. When it comes to microbes that have a lot of diversity or evolve quickly, memory B cells can be important because they can rapidly evolve antibodies to cover those new variants and refill the pool of LLPCs.




Can you give a reference for "With mice, even one dose of mRNA vaccine gives essentially permanent antibody titers at a very high level- this is not the case for humans and unfortunately we do not yet know why", please?
Excellent review, thank you. How did you find the actual peer review (was there a link from the original publication)?
Again, don't bring a mechanism to an epidemiology fight, right?
Bring a mechanism to an ongoing conversation, a research project, or an outcomes surveillance party!
(I've never been invited to an "outcomes surveillance party.")