Maternal Vaccination Part II: How Maternal Vaccination Works
Demystifying the lifesaving biology of vaccination during pregnancy.
In the first post of this series, we covered how maternal vaccination came to be, noting that it arose primarily because of the observation that pregnancy results in many infectious diseases becoming much more dangerous. In time, it became apparent that maternal vaccination could actually extend protection to the fetus and infant. This post delves into the biology of how that happens, what we still don’t understand, and touches upon some of the misconceptions behind how it all works.
Freshly delivered infants face a daunting problem: the world is full of pathogens, and their immune system is completely inexperienced in dealing with them. Fortunately, we’ve evolved a solution: maternal antibodies. How do they work? And how can we use vaccines to help improve upon their protection even further?
Antibody Transfer via the Placenta
Pregnancy allows for the transfer of antibodies from the circulation of the pregnant person to the fetus which will last for the first few months of life and protect against various infectious diseases that infants have never been exposed to, and thus have no immunological memory against. In this way, the immunological history of the pregnant person has a profound effect on the protection of the infant, as only antibodies against antigens previously encountered by the pregnant person can be passed on, and the levels of antibodies passed on are sensitive to the history of the pregnant person’s exposures. This naturally means that if antibody levels have waned for something for which there is a vaccine, the option exists to get a booster dose while pregnant (provided the vaccine can be given in pregnancy).
The antibody transfer across the placenta occurs through the neonatal Fc receptor (FcRn; this name has a convoluted history that misleads a bit about its role as the receptor has functions completely unrelated to the neonatal period) beginning at the ~17th week of gestation. This mechanism is summarized in the figure below.
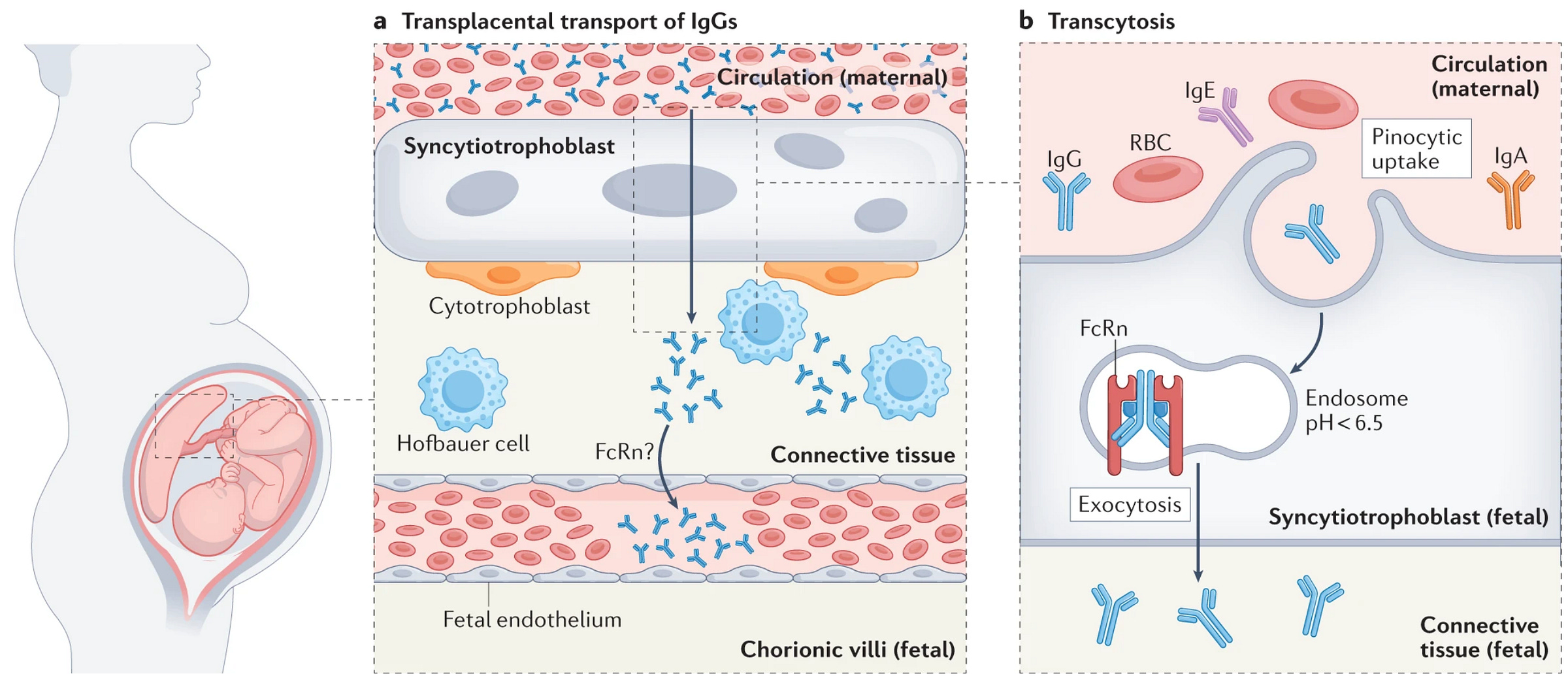
This is significant because it indicates that the transfer of immunity by maternal vaccination does not require any vaccines to pass across the placenta1. This means that a vaccine does not have to effectively induce immune responses in the fetus to be protective to the fetus. It also informs decisions about when to vaccinate against certain pathogens (pertussis and tetanus in particular), because vaccination can be timed to maximize the amount of antibody transferred to the fetus and therefore maximize the duration of protection (for other pathogens, their seasonal pattern of circulation is a more important determinant of the best time to vaccinate). In general, it appears that vaccination during the third trimester accomplishes this, but there are some considerations. For example, in the event of a premature delivery, earlier vaccination would be favorable because it would mean that at least some protective antibody elicited by vaccination were transferred, whereas vaccinating too late would mean only the baseline level of antibody in the mother would be passed.
In general, only IgG antibodies (the most abundant antibody class in the blood) are transferred across the placenta. The transfer ratio (the ratio of antibody in the blood of the pregnant person vs. cord blood) is typically greater than 1 for most vaccines, which has led many to conclude that IgG transport across the placenta is active (i.e. that antibodies do not just passively leak across the placenta but rather that they are pushed into the fetal circulation). Additionally, maternal antibodies in infants appear to have a longer half-life than for adults, but the mechanisms underlying this difference are not known. Transfer ratio also depends on term, with most antibody being transferred in the last 4 weeks of pregnancy:

As you can see from the figure above, IgG antibodies are not equally transferred across the placenta; human IgG has 4 subclasses (IgG1-4) with some subclasses transferring better than others (these will differ with the species; mouse IgG1 is not the same as human IgG1, for example).

The distinct subclasses of antibodies have different properties and functions.
Available data suggest that IgG1 and IgG4 are transferred the most efficiently, while IgG2 and IgG3 are transferred the least efficiently (except for R435H allotypes of IgG3). This has significance because different vaccine formulations can give different IgG subclass profiles, and so a vaccine which has a response that is biased towards e.g. IgG2 and IgG3 (depending on genetics) production may not be the best choice for maternal vaccination if the major intention is to protect the fetus/infant. The fact that IgG2 in particular is not well transferred across the placenta can be a problem for protection against encapsulated bacteria, as IgG2 is the major antibody induced to polysaccharide antigens (and furthermore children younger than about 2 years of age do not generate robust responses against polysaccharide antigens2). This is especially apparent in the case of Haemophilus influenzae type B, at one point a leading cause of both meningitis and epiglottitis (inflammation of the epiglottis, a flap covering the airway that prevents entry of food into the respiratory tract during swallowing; inflammation can cause marked narrowing of the airway and is life-threatening) in the US, until the introduction of the conjugate vaccine. Importantly, conjugate vaccines, though they contain the immunogenic polysaccharide of interest, are able to efficiently induce IgG1 which readily crosses the placenta and thus point to a strategy for protection against encapsulated bacteria in early life (however, transfer ratios may still be below 1). In another example of how these considerations may be applied, in one study, an RSV vaccine adjuvanted with alum was found to have poorer antibody transfer ratios compared with the unadjuvanted vaccine, which prompted that formulation to be abandoned (together with the observation that the immune response with the alum formulation did not appear to improve the overall antibody response against RSV F protein). Damage to the placenta can also compromise the transfer of antibodies, as has been seen in COVID-19, as well as malaria.
Some aspects of antibody transfer across the placenta are still contested; there is a general consensus that IgM antibodies cannot cross the placenta because they are too large and lack a specific receptor to do this, but there is some argument about whether IgE can cross the placenta (the major antibody class responsible for allergies). Nonetheless, there is so little IgE present in the serum (it is almost exclusively bound very tightly to mast cells) and it has a short half-life in the body (2-3 days), meaning that even if it can cross the placenta it is almost certainly biologically irrelevant (although the model described by Keith and Kabashima in the hyperlinked letter where it can hitchhike as part of immune complexes with IgG seems reasonable depending on the size and orientation of the antigen and explains how other non-IgG antibodies can be transferred across the placenta). However, because IgG is so much more abundant in the circulation than these other antibodies (and the import of these other antibodies is essentially an accident, per this model), direct consequences from this transfer are not likely to be important.
Another point that is contested is whether or not receptors other than FcRn (specifically Fc gamma receptors, FcγR) can contribute to the transfer of antibody across the placenta (there are many IgG receptors, but FcγRs induce signaling in the immune cells that express them and are not known to recycle IgG3). This is based on the observation in some studies that changes to the sugars added onto IgG can change the efficiency with which they are transferred across the placenta (pregnancy itself can dramatically change those sugars in a manner that is thought to reflect efforts by the immune system to tolerate the fetus), but this should not have any effect on binding to FcRn. On the other hand, sugars on the constant regions of IgG can affect the binding of IgG to certain FcγRs. This question is difficult to examine because there are many differences in placental structure and placental antibody transfer mechanisms by species (i.e. mice might not reflect what happens to humans even if they are engineered to express human Fc receptors). However, examination of paired samples of maternal and cord blood from human cohorts do not consistently show a meaningful difference in antibody transfer based on the sugars present on the antibody, arguing against a role for FcγRs in placental antibody transfer in humans. At the same time, the bias in IgG subclass does not precisely reflect the binding affinity for each subclass for FcRn as might be expected if FcRn were the sole determinant of placental antibody transfer (if this were the case, IgG3, rather than IgG2, would be expected to have the least efficient transfer). The literature on this subject continues to evolve. It nonetheless remains important to understand what factors determine the efficiency of antibody transfer across the placenta so that protection of infants can be maximized through maternal vaccination.
Amniotic Fluid Antibodies
The amniotic fluid contains antibodies, predominantly IgG, which seemingly come from fetal urine (just like most amniotic fluid). Their role in the protection of the fetus or infant is not well defined. Recent(ish)ly, however, it has been proposed that the antibody content of amniotic fluid can predict the development of severe respiratory syncytial virus (RSV) disease. Within the amniotic fluid, the fetus may ingest antibodies and these may readily enter the respiratory tract, where they may act to neutralize RSV upon exposure to the virus. However, the persistence of these antibodies at the mucosal surfaces of the respiratory tract is not known, and their role in protecting humans is far from established.
Maternal Vaccination and Human Milk
There are no vaccines routinely used today which rely on the induction of milk antibodies as a mechanism of protection. I mention this NOT for the purpose of discouraging breastfeeding but to address specifically a misconception I have encountered a lot: some who are hesitant to receive a vaccine in pregnancy would prefer to do it after delivery, with the intent that they will transfer the protective antibodies through breastmilk. While this necessarily means some delay (if brief) in the period in which an infant would be vulnerable to the disease in question, this is also not scientifically supportable- vaccines given in pregnancy are given in pregnancy for a reason.
While antibodies within milk can be protective against some infections (in particular those which target the gastrointestinal tract), their exact significance in protecting infants (as distinct from contributions from the antibodies within the circulation), especially with respect to respiratory infections, is incompletely understood (although it appears that in the case of pertussis, human milk is not sufficient for protection, and they do not appear to be sufficient to prevent infection by SARS-CoV-2, although the possibility that they may mitigate disease cannot be excluded). In large part, this knowledge gap is related to ethical constraints on study design (i.e., it would not be appropriate to randomize some children into breastfeeding vs formula feeding to study which group becomes sicker, although the PROBIT study has come close to this study design and has findings that largely agree with what might be expected from immunological first principles- fewer GI infections, lower risk of eczema, but no difference in respiratory infections for breastfed vs. formula-fed infants). Another factor to consider is that exclusive breastfeeding tends to be associated with other factors that promote good health e.g., maternal education level and socioeconomic status, which make it difficult to determine whether findings like those suggesting a protective effect from longer breastfeeding against influenza reflect actual biological realities or confounding. Again, this is not to say that breastfeeding lacks value, but that studying these questions is difficult and the field as a whole is not currently given adequate attention or funding or interest to be able to robustly answer these questions. Vaccination post-delivery should not be presumed to be an appropriate substitute for vaccination during pregnancy.
There are furthermore lingering open questions at the level of the basic immunology. For example, breastmilk comprises antibodies that are overwhelmingly comprise (secretory) IgA, which, in principle, should not be able to enter the circulation of the infant from the gastrointestinal tract, meaning that they would not be expected to confer protection outside the GI tract e.g. in the respiratory tract (this is not true of other animals, however). At the same time, some data suggests that these antibodies may end up in the circulation in at least some circumstances, although this is probably limited to very early in life. Some argue, however, that because of the immaturity of the infant digestive tract and their tendency to spit up what they consume, breastmilk does end up coating at least part of the upper respiratory mucous membranes, allowing it to offer at least some protection from respiratory infections. Studies disagree regarding whether human milk can interfere with the response to oral vaccines (rotavirus and polio), though the effect seems small regardless, but in any case, no recommendation to pause breastfeeding when oral vaccines are being administered exists currently.
Importantly, though the contribution of breastmilk antibodies to protection from various pathogens is incompletely understood, breastmilk does contain a number of other substances (e.g. lactoferrin, human milk oligosaccharides) which are believed to enhance infant resistance to infection and is in general a great way to meet infant nutritional needs (although some supplementation is still required, specifically with vitamin D and vitamin K, in exclusively breastfed infants). However, it may not be the best option for everyone and modern infant formulas are also an excellent option, provided that they can be safely prepared (i.e., there is access to clean water). Additionally, some individuals may be compelled to seek out donor milk to meet their child’s nutritional needs- please DO NOT do this without first speaking to your child’s pediatrician. Breastmilk can be a potential source of infection (such as HIV) or drugs and studies have shown risks with using anonymous donors from the internet. Milk banks in North America can be found here.
The totality of evidence suggests that, although breastfeeding may contribute to protection against a number of infectious diseases, for current vaccines, vaccination after delivery to induce breast milk antibodies is NOT an appropriate substitute for vaccination during pregnancy. It is, however, perhaps worth noting that although current vaccines are not known to work through the protection conferred through milk antibodies, the possibility that future vaccines could use this mechanism should not be excluded and further research in this arena deserves to be pursued given major outstanding questions of medical and public health importance.
Incidentally, fetal immune responses can be measured and detected: because IgM cannot cross the placenta, fetal antigen-specific IgM can be presumed to be the result of the fetus mounting a response against an antigen.
Polysaccharide antigens are unique in that their response does not require T cell help (they are formally known as TI-2 antigens, meaning T-independent type 2). T cells cannot sense carbohydrate antigens (with some exceptions) and thus cannot provide B cells that recognize polysaccharides with the help needed to make high affinity, class-switched antibody. Thus the response to polysaccharide vaccines is entirely (or almost entirely) B cell-intrinsic. However, attaching the polysaccharide of interest to a carrier protein (to form a conjugate vaccine) can allow B cells specific to the polysaccharide to take up the entire complex and present the protein component to T cells to get help. This phenomenon is called linked recognition:

Linked recognition requires that the two antigens in question be physically associated with one another. Presenting a polysaccharide with a carrier without physically linking (i.e. by covalent bonds) them will not produce linked recognition.
Most sources that discuss the lack of response to polysaccharide vaccines in young children attribute it to the immaturity of the splenic marginal zone (MZ), which contains the populations of MZ B cells that are responsible for responding to polysaccharide antigens. In general, before the age of 2 in most individuals, this part of the spleen is not well-developed and it takes until about the age of 5 to generate responses similar to adults. This explanation, however, has some inconsistencies. For example, a 23-valent pneumococcal vaccine administered to 12-month-olds elicited decent responses, so there is clearly some degree of biological variation in the responses across individuals (i.e. the threshold of 2 years is not an absolute rule). The key B cell population implicated here are the marginal zone (MZ) B cells- but these, contrary to their name, do not exist solely within the marginal zone and may be found in the subcapsular sinuses of lymph nodes (of which there are about 600), tonsillar crypts, and subepithelial areas of the mucosa-associated lymphatic tissue (MALT). It has also been found that, in humans, marginal zone B cells appear to recirculate rather than remaining in the spleen or lymphatic structures they normally reside in. Consistent with this, individuals who lack spleens are able to mount robust responses to polysaccharide vaccines (interestingly, the lack of MZ B cells has also frequently been attributed to the increased risk posed by encapsulated bacteria from these infections- this is actually more probably because of the absence of specific splenic macrophage populations which are especially good at clearing bacteria from the circulation that are not well-opsonized). For these observations to explain the apparent lack of effective response to polysaccharide antigens in infants, additional assumptions about the development of marginal zone B cells in humans are needed beyond the lack of a developed marginal zone in the spleen. Nonetheless, the finding that children typically need to be at least 2 years old for effective responses to polysaccharide vaccine is useful and actionable as it suggests other approaches for children younger than this, i.e., conjugate vaccines, maternal vaccination.
FcRn can support signaling by antibodies via Fcγ receptors through its action as a coreceptor, wherein it helps tether the antibody (or immune complex) alongside the Fcγ receptors, forming a ternary complex that presumably supports signaling:

However, to the best of my ability to tell, a direct role for FcRn in signaling (e.g., through ITAMs/ITIMs/ITSMs/ITAMis) has not been observed. Mutagenesis studies of the cytoplasmic domain of FcRn in mice have not demonstrated an effect on IgG metabolism.




Another excellent thread Edward!
Perfect!