Antivirals- things to know for the holiday season and beyond
I've gotten tons of messages lately about antivirals and I've been really alarmed by the misconceptions they reflect. Let's fix that.
Antivirals are a really important type of medication that, as their name suggests, work against viruses. The power of these medications can’t be overstated: they have taken HIV from a certain death sentence and made it a manageable chronic condition (provided that the medications can continue to be taken consistently) and they can even cure hepatitis C virus infection, which almost always becomes chronic hepatitis otherwise (but the financial cost of the antiviral regimen is unbelievable). There are also examples of antivirals for acute infections though, like Paxlovid (Nirmatrelvir/Ritonavir) for COVID-19 and Tamiflu (Oseltamivir) for flu. The trouble is: our tools are only as good as our usage of them. If we misuse these antivirals, it ends up being costly and unhelpful. Here, I will focus on the antiviral options for COVID-19 and flu (there are no routinely used antivirals for RSV1) explaining the things that everyone should know about them (and a few supplemental things but I will state in advance what is and isn’t important).
I will also preface this by noting that antivirals are NOT a substitute for vaccines or any other infection-prevention or mitigation measures. They are to be used when other interventions have failed to prevent infection in the patients in whom they are likely to have benefit and in the time period in which that benefit is likely.
If I could distill the essence of this (admittedly long) post into a few points though:
FOR COVID-19 AND FLU IN THE US: The Test2Treat program is in effect nationwide and can connect you to a telehealth provider who can help get you a free test and antiviral therapy (including a delivery) if it is appropriate, provided you are eligible for the program.
Antivirals don’t exist for most viral infections. Some notable exceptions include: COVID-19, influenza, hepatitis C virus, HIV, smallpox, mpox, vaccinia, chickenpox (and shingles) and other herpesviruses, etc. Because they do not exist for most viruses, it is often thought that it is not usually worthwhile to find out which specific virus is infecting you (unless an antiviral exists for it and you would be likely to benefit from it) as knowing will not change management of your illness.
You generally should not expect to take an antiviral and feel back to 100% basically immediately. With most viral infections (but obviously not all of them), you tend to get better on your own and the antivirals can speed this process up or prevent you from getting sicker, but it isn’t like a magic “off” switch for your symptoms. Sometimes just waiting to get better is the best way to go, unfortunately.
Antivirals should be taken as early into the illness as possible, even if you do not feel especially poorly- the point is to keep you from getting to the point of being very sick.
Antivirals should be taken as early into the illness as possible.
SERIOUSLY, ANTIVIRALS SHOULD BE TAKEN AS EARLY INTO AN ILLNESS AS POSSIBLE.
Some antivirals can be taken preventively if someone in your household has the infection to keep you from getting sick (specifically for flu- not really an option for COVID-19). You should ask about this, particularly if your household has people who are at high-risk from the virus in question.
An antiviral for your illness may exist- that doesn’t necessarily mean it is worthwhile for you to take it.
There are a lot of open questions about how well many of our antivirals work for different conditions that make it hard to tell whether they may be worthwhile for a given patient, for example, how well they prevent long COVID. We must demand more randomized controlled trials and require that all trial data from sponsors be shared, even if it is negative.
Antivirals can be expensive (though not always). It’s unfortunate that cost needs to be a consideration but it’s seriously worth considering if you’re not in a group that clearly benefits from use of the antiviral.
Overview
Broadly, antivirals can be grouped into a few categories. Most antivirals are virus-targeting antivirals (VTAs): they act on some step of the virus replication cycle to inhibit it and thereby reduce the amount of virus in the body until it can eventually be cleared by the immune system. These are contrasted with host-targeting (or host-directed) antivirals, which target the host’s cells to inhibit viral replication. For example, an antibody against CD4 called ibalizumab can be used in cases of HIV to prevent the virus from entering T cells by blocking the binding site needed for HIV entry. A similar approach is underway for SARS-like coronaviruses, which works by targeting the ACE2 protein used by many coronaviruses to enter cells (in a way that doesn’t interfere with the physiological function of ACE2).
Among VTAs, many options exist for which steps of virus replication can be targeted:

Viruses all have common steps in their replication. Viruses must enter cells, release their genome into the appropriate cellular compartment, replicate that genome, form new viral particles, and go on to infect new cells. Interfering with any of these steps can greatly slow production of viral particles and impede the progress of infections. Within these steps, there can be great variation. For example, some viruses have to integrate their genome into the host’s own DNA, like HIV. Some viruses encode multiple proteins on a single piece of mRNA (polyproteins), which are processed into the individual proteins the viruses need to function, like coronaviruses. Alternatively, some viruses encode their genome along multiple pieces, allowing for production of proteins without the need for a polyprotein to process, like influenza2. The list goes on.
Timing is Everything
One of the most important aspects of using antivirals is that the earlier they are taken, the more effective they tend to be. Put another way: antivirals should be started well before a person is seriously ill so that they do not get to the point of being seriously ill. This particular point has been alarming because I keep hearing from people that they tested positive for COVID-19 and their physicians said that they would not prescribe the antivirals at that moment but would consider it if they were to worsen. Assuming these remarks are to be taken at face value, they reflect a serious misunderstanding of the way antivirals work. It is of course possible that the people who reached out to me are simply not good candidates for antiviral therapy regardless of when it would be offered, but the rationale provided is alarming. The need to start antiviral treatment early is consistently reflected in their prescriber recommendations. For example:
Tamiflu (oseltamivir) must be started for influenza within 48 hours of the first symptoms for it to have benefit. This is supported by data from the 2009 influenza pandemic, in which delayed administration of Tamiflu was consistently associated with worse outcomes.
As an even more dramatic example, Tamiflu can be taken as post-exposure prophylaxis before symptoms occur if someone in the household develops influenza, and in randomized controlled trials has shown efficacy as high as 89% against developing clinical influenza.
It should not be assumed that any effective antiviral will work as post-exposure prophylaxis.
Paxlovid (nirmatrelvir/ritonavir) should be started within 5 days of the start of symptoms of COVID-19. Data from electronic medical records suggests that the earlier it is administered, the better, but the retrospective nature of the studies mean caution is warranted.
When used in hospitalized patients (even as a 9-day course), remdesivir may possibly improve survival (although that can’t be said definitively from this data) and it does shorten illness somewhat. When used in outpatients at high risk of progression to severe COVID-19 even as a 3-day course, there is a drastic reduction in the risk of hospitalization by 87%.
The relevant conclusion here is that if you are infected, even if you do not feel particularly poorly, but you are a candidate for an antiviral therapy (if you aren’t sure, ask the professional who manages your medical care- it’s their job to figure that out), do not wait until you feel very ill to get antiviral therapy. Additionally, this underscores the importance of knowing you are infected early on, and knowing what you are infected with as soon as possible (or at least, knowing you are infected by something that has a treatment).
Another point to be made here is that sometimes we are sickened not because of the virus itself but because of our response to the virus. For example, Epstein-Barr virus (EBV) causes infectious mononucleosis (mono; and a ton of other things), but it is generally not useful to treat mono with acyclovir, even though the virus is susceptible to it. This is because the illness of mono is driven by an immune response against the virus and the virus is not, in general, actively replicating during a case of mono. Therefore, targeting viral replication through acyclovir doesn’t do much to help. A similar issue exists with other viral illnesses, like COVID-19:

People generally do not become seriously ill until the second week, at which point the levels of virus are declining and tend to be very low- antiviral therapy at this point is generally not useful. Exceptions do exist- immunocompromised individuals for example can become seriously ill through virus-driven mechanisms rather than from the immune response and so antiviral therapy in such patients might be useful even if it is given later. It should also be noted that this diagram likely overestimates the asymptomatic period by deferring to earlier variants; the incubation period has become much shorter with newer ones, which can make it harder to time antivirals optimally as peak viral load can be attained earlier with respect to symptoms.
I will also note that the importance of timing is also reflected in the power of vaccination: an immune system that is primed to respond before any infection has occurred is in a much better position to control the infection than one that is not.
With that out of the way, I’d like to look at some specific antivirals.
COVID-19 Antivirals I: Paxlovid
I will spend the most time on this one simply because there is the most to say about it.
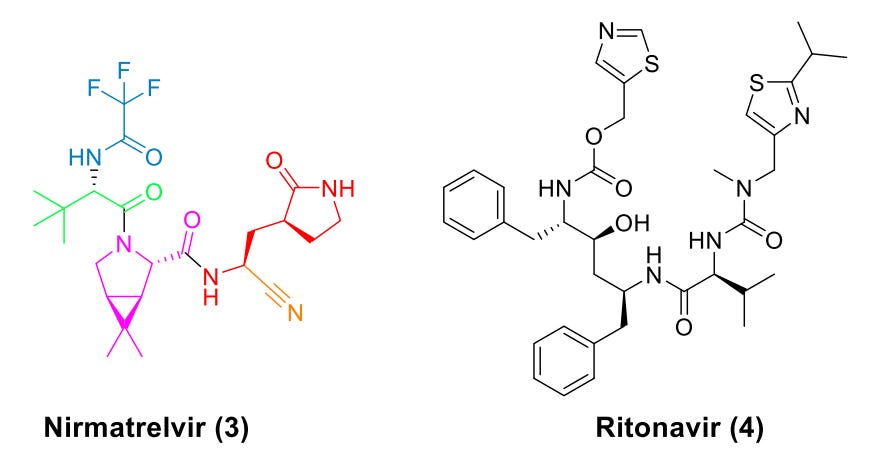
Paxlovid is the brand name for a drug comprising 2 medications: nirmatrelvir (NMV) and ritonavir (R), often written as “nirmatrelvir/ritonavir” or “N/R” or “NMV/R” to emphasize that they are taken together as a single drug:
Mechanism (just for fun- not stuff you need to know)
A lot of this section might read like gibberish to the average intelligent person who doesn’t have specific training in the field. I include this solely because I find the biochemistry interesting and I know some of the people who read my work like how I explain biochemistry. It is absolutely not necessary to understand anything in this part to know things that are important for your health regarding Paxlovid,so you can feel comfortable skipping it if that is your preference.
The antiviral effect of Paxlovid comes entirely from nirmatrelvir. Nirmatrelvir targets a protein in SARS-CoV-2 called the 3C-like protease (or main protease, MPro, also called nsp5). When SARS-CoV-2 (and other coronaviruses) replicate, they initially make polyproteins called pp1a and pp1ab which have to be processed into individual proteins. This is accomplished through the action of the proteins MPro and PLPro (but mostly MPro). Without MPro, the virus cannot effectively generate its replication machinery, and thus cannot productively be a virus. MPro first autocleaves itself from the polyprotein and then cleaves 11 more regions in the polyprotein to liberate the other proteins for coronavirus replication:

Nirmatrelvir interferes with this by engaging directly with the active site of MPro (i.e. it’s an orthosteric inhibitor). MPro is a cysteine protease with a catalytic dyad comprising a cysteine and a histidine, in which the imidazoline of the histidine abstracts a proton from the thiol of cysteine to form a nucleophilic thiolate. Nirmatrelvir’s nitrile group then acts as an electrophile to bond to MPro and prevent a catalytic cycle (i.e. processing of the polyproteins) from occurring. The resulting inhibition is reversible, but can still greatly slow replication of SARS-CoV-2. The inhibition is extremely potent with IC503 values of 4 nM.
The problem is that in the body, nirmatrelvir is metabolized too quickly on its own to get to levels that would exert an antiviral effect over a long enough time period to be useful. This is addressed with ritonavir, which inhibits an enzyme (CYP3A4) that metabolizes nirmatrelvir.
The Stuff You DO Need to Know
Paxlovid is given as a 5-day course taken twice a day (3 pills at a time- two 150-mg tablets of nirmatrelvir and one 100-mg tablet of ritonavir) for those who are at least 12 years old and weigh at least 40 kg with mild-to-moderate COVID-19 and symptoms beginning up to 5 days before starting the drug. It shows excellent results in those at heightened risk of developing severe COVID-19, as seen in the EPIC-HR study in which it showed a 88.9% reduction in the risk of hospitalization (7.01 % vs 0.77% in the placebo vs. Paxlovid group):
It is also noteworthy that 13 of the study participants died, all in the placebo group, further reinforcing that, when used correctly, the drug has a huge benefit to clinical outcomes. Paxlovid is the only clearly effective oral antiviral against SARS-CoV-2 currently available in much of the world (Japan also has ensitrelvir4).
The trouble is that the patient population this applies to, for the most part, no longer exists. When Paxlovid was trialed in those at standard risk in EPIC-SR, it did not have any clear benefit in reducing the risk of hospitalization from COVID-19. It also does not have an obvious effect in reducing hospitalization among vaccinated individuals, attributed to the fact that vaccination already lowers the risk of hospitalization so much that any additional benefit is very hard to capture if, it exists at all. In data from Israel, a benefit is not clearly apparent in those aged 40-64 (even though in the older age group benefit is huge and appears to show up before the drug even has a chance to work). It is still probably reasonable to offer the medication to those at very high risk even with vaccination, however.
Paxlovid has not been shown to reduce the risk of contracting COVID-19 when given as post-exposure prophylaxis, unfortunately (this is true of all antivirals currently available for COVID-19).
The outcome that is perhaps of greatest interest today with respect to Paxlovid however is long COVID. Long COVID is an extremely difficult condition to study because it encompasses what are likely many different conditions that we all call long COVID, which means that a particular intervention may have value for some of them but not all of them that we might miss because we are looking at all of them together. A large study from the VA looking at 280,000 people suggests that Paxlovid might lower the risk of long COVID in those with at least 1 risk factor for developing severe COVID-19, but another VA study found only a benefit for the risk of thromboembolic events (i.e. blood clots) from taking Paxlovid. In other words, it is not clear whether or not Paxlovid has a true benefit for lowering the risk of long COVID.
However, a major problem with much of the data here is that it does not come from randomized controlled trials. While these are definitely not the only way to get good data on whether or not a drug works, we have previously seen that the benefit of Paxlovid in retrospective studies (such as these) appears before the drug has really had any chance to work, suggesting that the people who get Paxlovid tend to be at lower risk at baseline (an example of immortal time bias). This makes it very hard to tell whether or not Paxlovid is actually helping with the risk of any of these outcomes.
Another point that is really important with Paxlovid, possibly the most important point: the ritonavir in Paxlovid is required for the medicine to work. Without it, nirmatrelvir gets metabolized to an inactive form too quickly and thus would not be able to help control the infection. Ritonavir inhibits CYP3A4 (and some related enzymes to a lesser extent), and also induces CYP3A4 (but to a lesser extent than it inhibits it) and several other enzymes involved in drug metabolism. CYP3A4 metabolizes more than 50% of all medicines, which means inhibiting it can slow their metabolism. The consequence of this is that the levels of those drugs while taking something with ritonavir may rise to toxic levels (or more rarely, may drop to inappropriately low levels if CYP3A4 activates the drugs from an inactive or less active form). Increases in the concentration of medications in the blood as high as 20-fold have been reported for some medications taken with ritonavir. It is absolutely critical to know what medications you are on (including herbs and supplements) before taking Paxlovid to confirm that it is safe to do so. The Liverpool Interaction Checker is a great resource to check medications for interactions. Fortunately, if toxicity occurs, it is correctable through the use of medications that (strongly) induce CYP3A4, like rifampin. Nonetheless, this would also mean that the nirmatrelvir would be metabolized at an accelerated rate, causing a loss of its antiviral effect.
Many people who take Paxlovid experience a bitter, metallic taste that has been dubbed by some as “Paxlovid mouth.” This is unpleasant, but basically harmless. Paxlovid should not be stopped because of this side effect.
Paxlovid use in those with kidney disease also deserves notice. Those who have eGFRs5 ≥ 30 to < 60 mL/min should take one nirmatrelvir pill instead of two (150 mg instead of 300) for each dose as their reduced kidney function will help ensure high enough levels of the drug at a lower dose. Unfortunately, those who have eGFRs < 30 mL/min are not currently recommended to take Paxlovid because of the toxicity risks involved and alternative antivirals (molnupiravir) should be used instead given the considerable risk from COVID-19 in such patients6.
Rebound Infections
Rebound COVID-19 basically describes a situation in which a person apparently recovers from COVID-19, then begins to experience symptoms again after a few days (the median time to rebound after starting antiviral therapy seems to be 12 days) and seemingly has to go through a second round with the virus. This state was originally defined by the presence of symptoms but some have used it to refer to the state of having a positive rapid test again even if symptoms are absent. For a while it was hard to tell whether or not Paxlovid truly increased the risk of rebound COVID-19, as it can occur even without any antivirals, but the data to date do make it clear that this risk is indeed increased through the use of Paxlovid (but the extent to which this happens varies widely from study to study, in part because of differences in how rebound is defined, but also the characteristics of the patient population). Importantly, individuals who experience rebound infections should be presumed to be contagious, as replication-competent virus has been cultured from them.
The cause of this rebound phenomenon is not well understood but multiple ideas are proposed. One hypothesis is the course of Paxlovid fails to completely clear the virus and then as it is discontinued and the immune response relaxes after virus has declined to levels less than those needed to stimulate a response, virus replication resumes. It has alternatively been suggested that the cause is an overexcited immune response against the virus whose replication has been suppressed by Paxlovid, but I find this explanation to be tenuous and as far as I can tell, the entire basis for it is that people who experience rebound tend to have more robust immune responses against the virus. It has been suggested that this might be avoidable if the course of Paxlovid were extended beyond 5 days but there are no specific data available to confirm or refute this. Some limited data also argues that the risk of rebound is higher when Paxlovid is started earlier but the number of individuals in the study in question is too small to make these conclusions firmly, and, critically, given that much larger datasets show that earlier initiation of Paxlovid seems to be more effective in preventing hospitalization (the fundamental reason you would prescribe Paxlovid), this should not be used as a reason to delay Paxlovid at this time. Most recently, a marvelously simple explanation has been put forth (it is my favorite so far, credit to Chris J. Maddison for bringing the preprint to my attention): in essence, the MPro inhibitors arrest replication of the virus and create an opportunity for it to be rapidly cleared by the immune system. However, the MPro inhibitors have a short lifetime in vivo, so once they are stopped, the drugs are eliminated, and polyproteins from the initial infection that linger “reanimate,” inducing new replication cycles. This explanation is the first one I have seen that seems to make perfect sense and explains why this seems to be uniquely common among MPro inhibitors and not other antivirals (rebound can occur with any of them, but seems to be more common with Paxlovid specifically). This work suggests that extending the duration of MPro inhibitor therapy by 3-5 days would reduce the likelihood of rebound by 8-to-32-fold. Alternatively, I would argue that rather than extending the course, MPro inhibitors could get around this by (1) being irreversible (i.e. make them inactivators rather than inhibitors- apparently this takes only a minor chemical tweak) or (2) having an additional moiety to use PROTACs to actively degrade the viral polyproteins via the proteasome (but these approaches require new drugs rather than being able to use old ones).
Regardless, here’s what needs to be understood about rebound infections:
It is not because of a poor immune response or the development of resistance to Paxlovid (or, more properly, resistance to nirmatrelvir7).
Rebound infections are not associated with hospitalizations- the antiviral still does what it was designed to do: keep people out of the hospital.
The illness and rise in viral load tend to be short-lived: a median of 3 days.
Rebound infections do appear to boost the immune response, helping to make you more prepared for your next encounter with SARS-CoV-2.
A second course of Paxlovid or other antiviral therapy is generally not recommended for rebound cases because they tend to be mild or asymptomatic (but this will depend on the patient in question).
Rebound infections are likely to be contagious.
Paxlovid Bottom Line
Paxlovid is extremely effective in preventing hospitalizations from COVID-19 in unvaccinated patients who are at heightened risk for progressing to severe disease, with earlier initiation of courses being better (I cannot stress this enough: antivirals should be taken as early into infection as possible- literally as soon as you have a positive test result). Its use in other patient populations is unclear in terms of the benefit offered, particularly in vaccinated people at low risk. Similarly, its effects in preventing long COVID are not clear. The risk of rebound infection with Paxlovid exists (as it does with COVID-19 in general), but should not be used as a reason to not take the medication in those at heightened risk for poor outcomes. Paxlovid also comes with major challenges in some patients with regard to managing drug interactions. We urgently need more randomized controlled trial data for Paxlovid in patient populations relevant to today’s landscape of widespread immunity.
COVID Antivirals II: Lagevrio (Molnupiravir)

If it’s hard to tell whether Paxlovid will be useful in a given patient, the value of molnupiravir is even more opaque. Molnupiravir is taken orally as an 800 mg (four 200-mg pills) dose twice a day for 5 days within 5 days of symptom onset.
Molnupiravir works on the principle of error catastrophe. As living things (and viruses) replicate their genomes, errors occur in the copying process. Most of the time, these mutations are useless or harmful. In rare cases, they can confer a benefit, and in those instances, will be selected for by evolutionary forces: the virus that has an advantage over the other viruses will outcompete the other viruses for resources and replicate and spread to a greater extent. However, there is a limited tolerance for mutations during any given replication cycle: make too many errors, and the genome becomes a mess that fails to produce functional products. This is known as error catastrophe:

Molnupiravir works by drastically accelerating the rate of these mutations to make SARS-CoV-2’s genome an irreparable mess (i.e. making you go from the white region into the shaded region above):

In the body, molnupiravir is converted to NHC, which directly substitutes for the base C and can specify either an A or a G in the product RNA it templates. Animal and in vitro data showed that after molnupiravir is introduced to infected animals and cells, virus rapidly disappears- in fact, in animals, molnupiravir, but NOT Paxlovid, prevents transmission of SARS-CoV-2. Merck initially reported a 50% reduction in the risk of hospitalization from their Phase 3 trial based on interim data, which was very exciting. However, over time, cold water was thrown on that number, with the full data of the trial showing a 31% risk reduction for hospitalization with molnupiravir, but even more surprisingly, in those who had immunity it seemed that the placebo group may have done slightly better than those who got molnupiravir (but the small numbers here mean that differences could be attributable to random chance). What’s more is that while error catastrophe as an antiviral mechanism certainly makes sense, it is likely that the extent to which coronaviruses are near their error threshold (the critical frequency of errors that renders them incapable of producing viable infectious particles) has been overestimated, as coronavirologist @wanderer_jasnah explained so eloquently. In fact, since its approval, a lineage of SARS-CoV-2 bearing a unique mutational signature suggestive of molnupiravir use has been found, raising concerns that molnupiravir might give rise to novel variants of SARS-CoV-2.
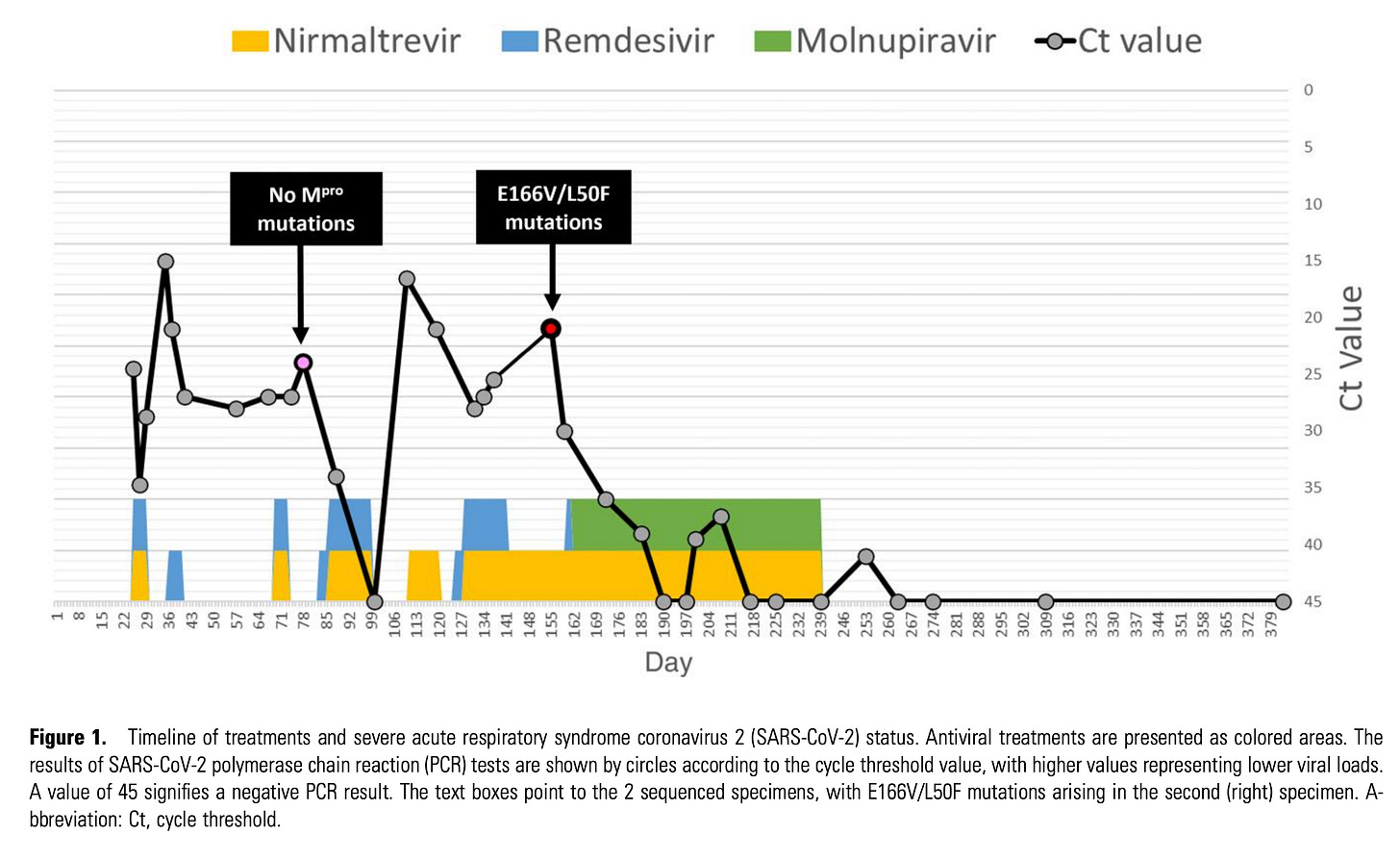
Since the original pre-approval data, we have data from the PANORAMIC (open label) randomized controlled trial suggesting that molnupiravir doesn’t reduce hospitalization in vaccinated adults, though the drug was safe and patients who took it reported feeling better more quickly than those who didn’t. Molnupiravir might reduce the risk of long COVID slightly, but it’s not clear. One finding that is very interesting however comes from a case report of an older adult male with follicular lymphoma who had multiple extended courses of Remdesivir and Nirmatrelvir/Ritonavir but continuously failed to clear virus until molnupiravir was added to his antiviral regimen:
This is paired with another case report and review summarizing the combined use of molnupiravir and nirmatrelvir/ritonavir suggesting that the combination may have value in immunocompromised patients (but we lack high-quality, prospective, randomized trial data to say this with confidence- we should get on that).
Lastly, it should be noted that molnupiravir is not recommended in pregnancy because of the risk of birth defects seen in preclinical models and if given to males it is recommended that they do not have unprotected sex for 3 months; however, in the gold-standard tests for mutagenic potential in humans, molnupiravir does not show a risk. There are also concerns about its use in children <18 years old because of the risk of effects on bone or cartilage growth in animals.
Molnupiravir Bottom Line
Molnupiravir’s effectiveness in any group today for basically any outcome is not clear and it may contribute to the development of novel variants of SARS-CoV-2 (although these variants may not necessarily have any kind of fitness advantage that would extend their public health importance). In high-risk patients where there is no suitable alternative treatment, it might make sense. It may have an adjunctive role when used with other antivirals in immunocompromised patients but this is not well established. Alternatives should be considered in individuals of childbearing potential and children younger than 18 (and basically everyone else).
COVID-19 Antivirals III: Remdesivir

Remdesivir was the first COVID-19 antiviral to be introduced. The major limitation of remdesivir is the way it is administered: it has to be given as an IV infusion over 3 days with an initial dose of 200 mg on day 1 and then 100 mg on days 2 and 3 within 7 days of symptom onset in those as young as 28 days old. This is because it is not effectively absorbed by the oral route. Despite that, there is basically no doubt that remdesivir is extremely effective in preventing hospitalization among those at increased risk for it without immunity. Early trials of remdesivir in hospitalized patients (as a 10-day course) suggested that it shortened the duration of illness and might have a benefit in promoting survival (but it was not clearly there in the data- the benefit seemed to be greatest in those who had the lowest oxygen requirements). Still, because a benefit was seen in hospitalized patients, remdesivir is recommended in hospitalized patients over no antiviral at all. If you’ve been paying attention, you know why the results were so lackluster already: antivirals need to be given as early as possible. When the PINETREE trial came out, which targeted outpatients at high risk of bad outcomes from COVID-19, remdesivir gave a whopping 87% lower risk of hospitalization or death compared with placebo. Furthermore, unlike Paxlovid, remdesivir doesn’t come with a laundry list of concerning drug interactions (but you still can’t give it to people with eGFRs < 30 mL/min).
As far as mechanism, remdesivir is a bit of a weirdo- similar to molnupiravir, it mimics a component needed for SARS-CoV-2 to replicate its genome (in this case adenosine whereas molnupiravir’s active metabolite mimics cytidine/uridine), but it stops replication of the RNA strand 3 nucleotides after it gets introduced (this is known as chain termination).

It’s also worth mentioning that because remdesivir is not absorbed well when taken orally, there are next-generation antivirals that build upon it that can be taken orally such as obeldesivir and VV1168. Victoria Yan’s writing on this is a must-read.
Remdesivir unfortunately suffers from the same data gaps we have for other antivirals. We do have an RCT looking at long COVID, but it has no clear evidence of remdesivir being helpful, although this looked specifically at hospitalized patients. We have no data to inform its use in vaccinated individuals. As for safety, remdesivir can occasionally cause elevation of biomarkers suggestive of liver inflammation but the rise is not substantial and does not have any clearly negative health consequences. Remdesivir has been rarely reported to cause a slowing of the heart rate (sinus bradycardia), the causation for which seems plausible because the medication (by design) resembles adenosine (triphosphate) and adenosine slows conduction in the heart.
There is one bizarre myth I keep encountering that says remdesivir is some wildly dangerous medication that kills people; As far as I can tell this comes from a trial that tested remdesivir for Ebola virus disease (EVD) alongside multiple other interventions that we now know do work against EVD. Remdesivir does not work against EVD (unfortunately) and EVD has a very high case-fatality ratio, so the result is that the group that got remdesivir and ZMapp fared the poorest given that the other interventions are effective. In short, don’t give remdesivir for EVD, but the medication in general appears to be safe.
Remdesivir Bottom Line
While it seems to have comparable effectiveness to Paxlovid but without the complex drug interactions and it can be used in those as young as 28 days old, the fact that it has to be given intravenously over multiple days hampers the use of remdesivir greatly. It’s also among the only antivirals that can be used in hospitalized patients per guidelines. However, like Paxlovid, it also can’t be used in those with very poor kidney function (eGFR < 30 mL/min). In addition to these, we lack a lot of data on how well remdesivir works in the patient populations it is most relevant in today and how well it works against long COVID. In situations where an antiviral is warranted, remdesivir is a strong alternative to Paxlovid, provided the hurdles of administration can be cleared.
COVID-19 Antivirals IV: Metformin
One of the most interesting findings to come out recently is a randomized controlled trial (COVID-OUT) showing a 41% risk reduction in the (self-reported9) risk of long COVID from 14 days of early metformin treatment:

If this result holds, this would make metformin the most effective therapeutic to prevent long COVID that we currently have. The study seems well matched regarding the characteristics of metformin vs. placebo, though the cohort tends to have a relatively high rate of obesity and the metformin group was slightly more likely to have been vaccinated or receive a booster vaccine dose. Most of the data come from the delta variant period. Importantly, when looking specifically at vaccinated people, metformin does not have a clear benefit:
This again reinforces that vaccination is probably the most powerful tool we have against long COVID and all of COVID’s badness in general- it will probably be difficult to show a benefit on top of it.
Metformin has many advantages as a medication: it’s cheap, accessible, taken orally, and exists in a generic form. However, the medication’s fundamental purpose is actually as a treatment for type 2 diabetes (although it does have other uses). Metformin has a complex mechanism of action, but most of the effect seems to come from its ability to inhibit gluconeogenesis- a metabolic pathway in which amino acids are shunted to make energy- and stimulation of AMPK (AMP-dependent protein kinase), mainly acting in the liver. The consequences of these changes are that the body shifts to breaking down fats (rather than amino acids) and carbohydrates to meet energy demands. Metformin also seems to have complex effects on the immune system that would not be practical to summarize here for reasons of length.
However, when I asked Professor Boulware, an investigator on the study, about how metformin might be achieving this result, I was directed to a study (preprint) which showed that metformin seems to have an antiviral effect against SARS-CoV-2 as those in the COVID-OUT study who received metformin had consistently lower viral loads after starting the medication compared with placebo:
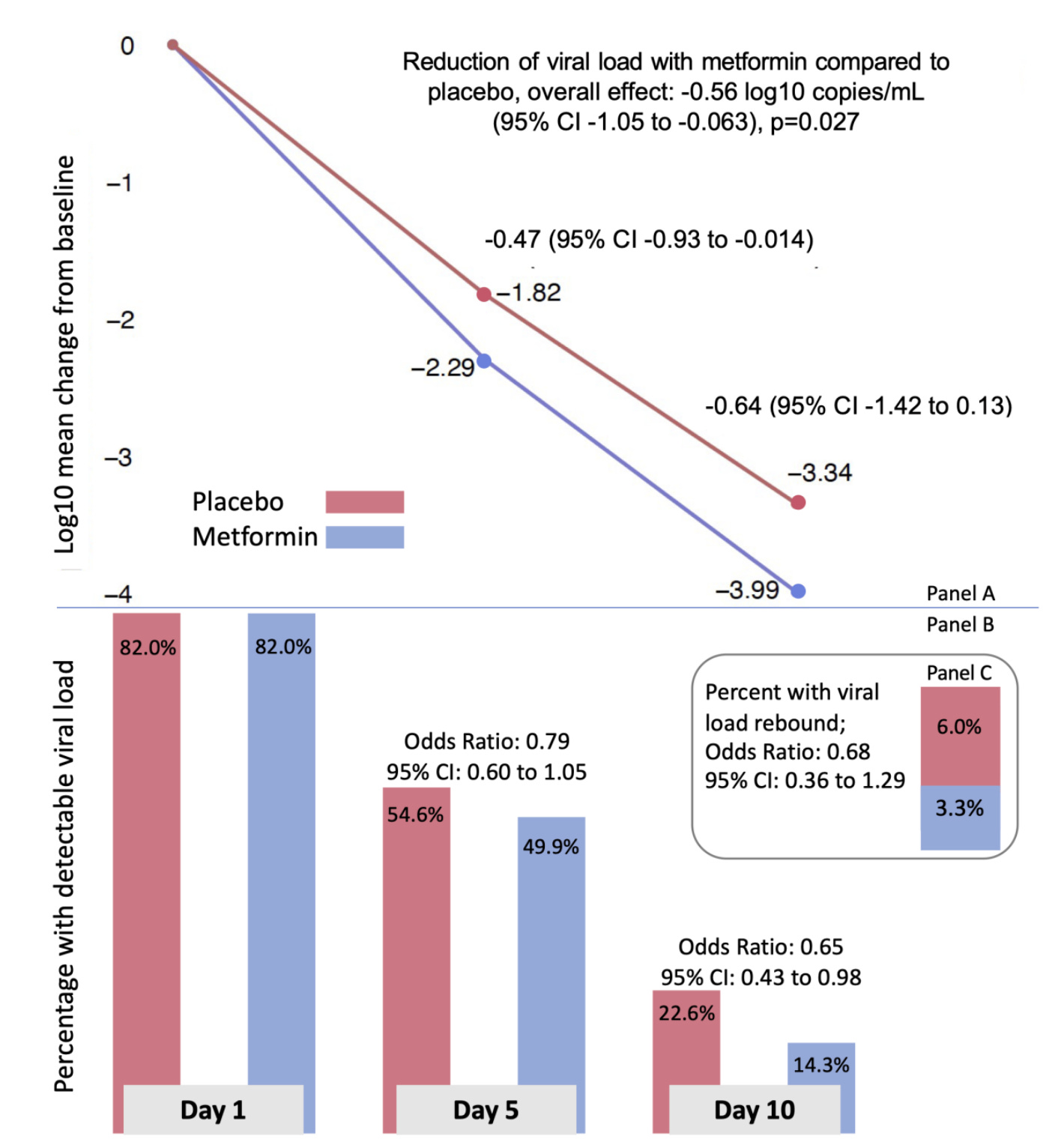
In vitro data also supports that metformin has an antiviral effect against SARS-CoV-2.
Despite this, the TOGETHER trial did not show a benefit for metformin for the risk of hospitalization, but this might be explained by the fact that the adherence to metformin was very poor among study participants because they were immediately started on 1500 mg instead of working up to the dose, causing unpleasant side effects. In contrast, the COVID-OUT study used 500 mg once a day on day 1, twice a day on days 2-5, and then 500 mg in the morning and 1000 mg in the evening to day 14. Interestingly, the TOGETHER trial also showed a benefit for fluvoxamine which was not reproduced against long COVID in the COVID-OUT trial. Guidelines for fluvoxamine use in COVID-19 advise against its use outside of a clinical trial because of inconsistencies across study results. There are currently no specific guidelines to inform use of metformin in COVID-19, and, fortunately, the risk of long COVID has been declining over time. Because of this, it’s hard to know where to place metformin in our therapeutic options for COVID-19.
Supplementary: COVID-19 Antivirals V: Interferon λ
While not currently available in the US as a drug, interferon λ has uniquely shown clear benefit in reducing the risk of hospitalization and emergency department visit in vaccinated individuals against COVID-19 when given as a single subcutaneous injection at 180 micrograms within 7 days of symptom onset (although benefit is only clearly apparent when symptoms occurred 3 days before or earlier- what was that I said about when you’re supposed to give antivirals? I forget.):

Interferon λ (also known as type III interferon) is a natural component of our own immune system which acts mainly at the mucosal surfaces. Unlike other classes of interferon, interferon λ is commonly summarized as having antiviral effects without provoking inflammation, which makes it attractive as a drug (but this is an oversimplification). Still, not all of the data on interferon λ is positive with other studies reporting no clear benefit. It is proposed that the dose of interferon λ in this negative study is too low- though notably, it is the same dose and route as used in the TOGETHER trial which did show the positive result. It is preferable not to give multiple doses or use higher doses because of a risk of a documented prior risk of liver toxicity (although, notably, this study uses the drug to treat hepatitis C virus and an elevation in liver enzymes may reflect more efficient killing of infected cells). It is conceivable that localized delivery of interferon λ to the respiratory tract might give better results. Anyway, this is all still theoretical because the drug is not approved.
Summary of COVID-19 Management Options
A really excellent recent review has a great table summarizing how to approach treatment of COVID-19 in light of all the available evidence at this time:
Detailed guidelines from the IDSA can be found here.
Influenza Antivirals I: Oseltamivir and other Neuraminidase Inhibitors

There are basically two types of antivirals that are available for influenza10. The first of these are neuraminidase inhibitors (NAIs).

Influenza enters cells through its hemagglutinin protein which recognizes sheets of a sugar called sialic acid on the surface of cells. To exit them however, it needs to shear off these sialic acid residues with an enzyme called neuraminidase (aka sialidase). If it cannot do this, influenza gets stuck on the surface of the cell it infected and cannot spread and replicate. Neuraminidase inhibitors work by resembling sialic acid and clogging up the enzyme, preventing influenza from being able to leave the surface. The major neuraminidase inhibitors are oseltamivir, peramivir, and zanamivir. Of these, only oseltamivir (Tamiflu) can be taken orally. There is a lot of data controversy regarding oseltamivir and the real odyssey is very convoluted and involves multiple bad actors, not all of whom are Roche, the manufacturer of Tamiflu (maybe I’ll dive into this more in a future post but it’s not practical to get into right here). The upshot of it is that we do not have adequate randomized controlled trial data to tell us about how well oseltamivir works in reducing hospitalizations from influenza (or how helpful it is in patients who are hospitalized with influenza), which, as discussed earlier, is a major issue in understanding how to best use the drug. In a recent editorial, Tim Uyeki (Chief Medical Officer of the CDC’s Influenza Division), together with David S. C. Hui and Nelson Lee explain that at this point, to tell whether or not there is a benefit for the use of neuraminidase inhibitors against the risk of hospitalization would require a randomized controlled trial of a minimum of 15 000 to 30 000 participants, something that no one has yet put forward the money to examine (which is horrible).
Nonetheless, from randomized controlled trials we see:
Oseltamivir is very effective (84% relative risk reduction for households, 89% for individuals) in preventing influenza when given as post-exposure prophylaxis to members of the household.
Oseltamivir shortens the duration of illness from influenza by about a day in low-risk individuals and about 2-3 days in higher risk individuals.
Oseltamivir shortens the duration of illness by about a day in unvaccinated adults and reduces illness severity, but increases the risk of nausea and vomiting.
In children aged 1-3, it shortens the duration of illness by 3-4 days when started within 24 hours of symptom onset and reduced the risk of middle ear infections by 85% when given within 12 hours of symptom onset .
Use of oseltamivir in healthy and at-risk adults and adolescents reduces the use of lower respiratory tract illness (e.g. pneumonia), antibiotic use, and hospitalization.
Long-term use of oseltamivir for 6 weeks in older adults, both vaccinated or unvaccinated, lowers the risk of laboratory-confirmed influenza by 92%.
While it is definitely very rare, current IDSA guidelines do support the use of oseltamivir preventively in a limited subset of patients who have very high risk from influenza and/or are unlikely to develop effective responses to vaccine.
A larger RCT also found a significant protective effect (87%) from oseltamivir when given preventively during the peak of flu season in preventing clinical influenza.
28 days of inhaled zanamivir in adults and adolescents at high risk of complications from influenza demonstrated an 88% lower risk of laboratory-confirmed influenza relative to placebo.
Aside from these, it is considered standard of care to give NAIs in hospitalized cases of influenza. As you might guess, the value of this is really unclear, but observational data suggest that giving the NAIs earlier in the course of hospitalization seems to be better than giving it later (or not at all).
Because it’s the one that’s taken orally, oseltamivir tends to be the most commonly used NAI. Zanamivir (Relenza) is given by inhalation and seems to have a meaningfully higher barrier to resistance than oseltamivir (and strains resistant to oseltamivir are usually not resistant to zanamivir). However, zanamivir tends to have some issues in those who have respiratory disease like asthma, as it can cause bronchospasm (which makes it challenging to analyze observational studies comparing it to other antivirals because these conditions put a person at higher risk of influenza, meaning those doing better on zanamivir might have done better without it). The barrier to resistance for NAIs as a class is relatively high, although in the past, oseltamivir resistant isolates of influenza were found sporadically11; the CDC monitors circulating influenza viruses for drug resistance and thus far the picture is reassuring.
Oseltamivir is taken as a pill or suspension if swallowing pills is a problem. Oseltamivir mainly has the issue of causing nausea and vomiting, but this generally seems to improve if it is taken with food and tends to resolve over the course of taking the medication. There are also some data that Oseltamivir can cause neuropsychiatric adverse events (though this doesn’t seem especially common), but the extent to which this is a true risk of the drugs (as opposed to an effect of influenza) is debated.
Neuraminidase Inhibitors Bottom Line
Influenza is very challenging to target with antivirals because it replicates so quickly that timing an antiviral in the window where it would actually help is very difficult (people typically don’t know what they have is influenza or they think they’re well enough that they don’t need treatment even though a few days later they will feel like they were hit by a train; also rapid tests for influenza are lacking). In general, NAIs are a safe option for the treatment of influenza, reliably shortening the duration of illness and likely reducing the risk of hospitalization in those at increased risk for it, if you get them early enough (ideally within 48 hours of symptom onset, but sooner does seem to be better). The major place where NAIs are probably most helpful and most underused is as prevention after exposure or if taken long-term for those who meet criteria. Many open questions exist about what the exact value of NAIs is in the management of influenza in large part because people don’t seem able or willing to organize a randomized controlled trial of appropriate size to figure this out.
Influenza Antivirals II: Baloxavir Marboxil
Baloxavir marboxil is the newest influenza antiviral, first becoming available in the US in 2018. It works by stopping a process called “cap snatching.” When our cells make mRNA, they require a cap to be able to exit the nucleus and be translated into a protein. Influenza RNAs do not have caps: they have to steal them from our own RNA transcripts, which happens through the action of influenza’s PA (polymerase acidic) endonuclease protein. Balovavir inhibits this process, thereby making it much more difficult for influenza virus to make RNAs that can serve as templates for influenza protein production. Baloxavir is also nice in that you only need to take a single oral dose once for the entire course (but you have to be at least 12 years old).
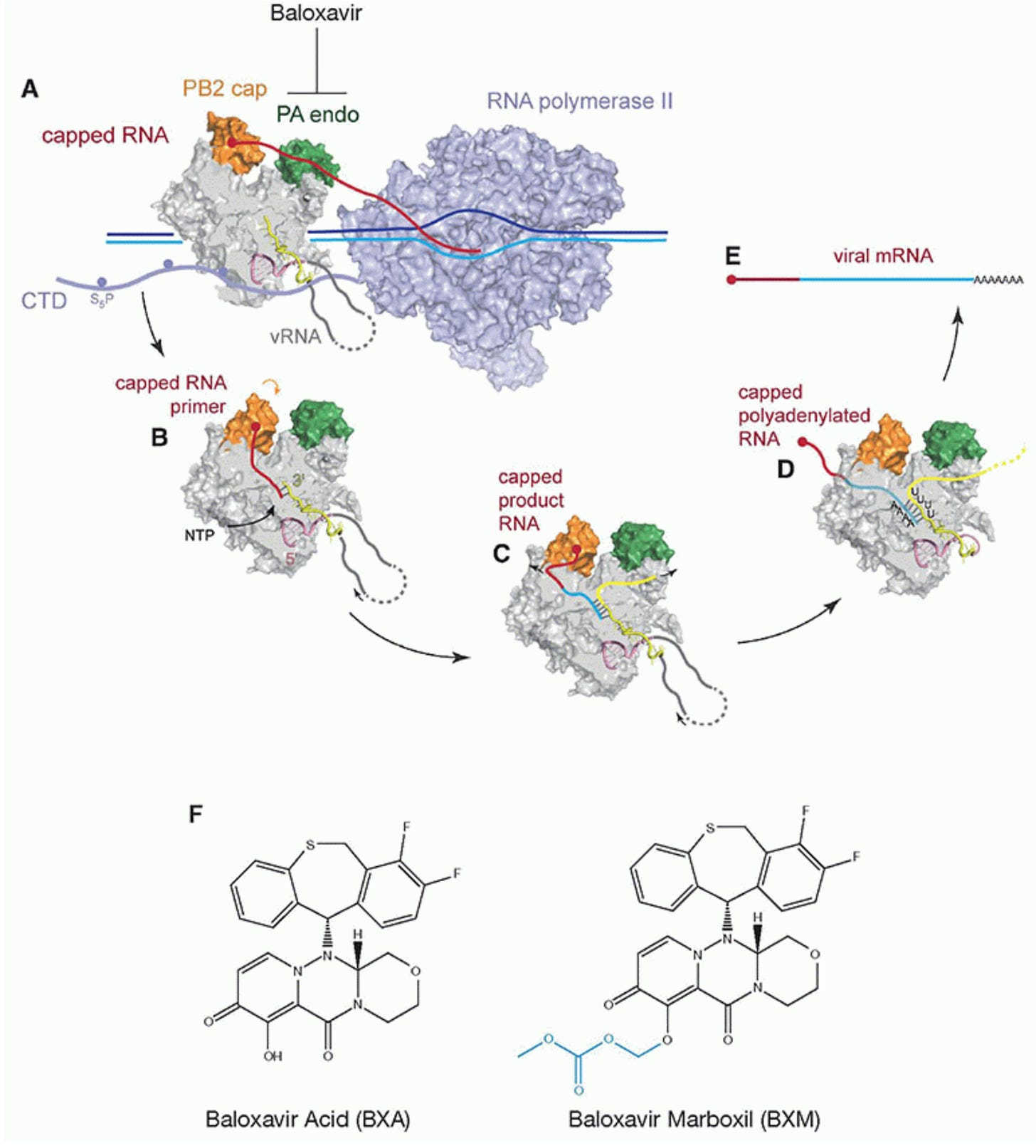
However, like NAIs, baloxavir has to be taken within 48 hours of symptom onset. Also, unfortunately, the barrier to resistance for baloxavir seems to be lower than for NAIs (but resistance in current isolates is still uncommon). There are currently concerns about how long baloxavir can continue to be used given this issue (but for now, it’s definitely viable). Because baloxavir is a relatively newer drug, there is less clinical data to draw upon for it but from the randomized controlled trials, we know:
Baloxavir relieves symptoms more quickly than placebo to a level comparable to oseltamivir, but seemingly reduces viral load more than oseltamivir.
Baloxavir lowers the risk of developing influenza after exposure by 57% (which is less than what RCTs for neuraminidase inhibitors tend to show but given different patient populations and different time periods, it’s hard to compare directly; in a retrospective study at a single center, baloxavir and oseltamivir worked similarly well to prevent infections within the household)
In children aged 1-12 (note that baloxavir isn’t approved for this age group), baloxavir reduced shortened the duration of illness to a similar extent as oseltamivir and was well tolerated
In high-risk adults and adolescents as young as 12, baloxavir outperformed oseltamivir in reducing symptoms of influenza, viral load, and had similar fewer serious adverse events than the placebo group
In addition to this, observational data suggest it may reduce hospitalizations more than NAIs, but this needs validation in randomized controlled trials, and other observational data suggest it is generally similar to NAIs. Baloxavir is also reported to reduce healthcare utilization and cost much more than NAIs, especially in high-risk patients.
Baloxavir Bottom Line
Baloxavir is an effective antiviral that can be given orally as a single dose (making it super convenient) for influenza either to shorten the duration of symptoms or to prevent development of influenza after exposure provided you are at least 12 years old. It seems to work similarly well to NAIs but it tends to be better tolerated (nausea doesn’t seem to be any more common than placebo). We lack randomized controlled trial data on how well it reduces hospitalization or death from flu (which is the biggest motivation for taking the antivirals in the first place), but by most metrics, seems to be at least as good as NAIs. The major concern with baloxavir is that resistance tends to evolve relatively easily- but currently, resistant isolates of influenza are rare, meaning the drug should work fine- provided you take it early enough.
In rare cases, ribavirin can be used for RSV but this medication does not show a clear benefit in terms of outcomes. There are monoclonal antibodies for RSV, as well as vaccines for pregnant people and older adults which are highly effective in reducing the risk of hospitalization but they are not given as treatment.
Influenza can also encode additional gene products through the use of alternative reading frames and splicing.
IC50s are a metric in pharmacology and related disciplines which describe the amount of a substance you need to reduce the activity of an enzyme by half. The lower an IC50 value, the more potent the inhibitor is. There is a rule of thumb that to block 90% of the activity of the enzyme (IC90), you need 10 times the IC50, and to block 99% (IC99) you need 100 times the IC50. In general, for (small molecule) drugs to be therapeutically viable, IC50 values need to be in the nanomolar (nM) range or lower. When exceeding this value, the amount of drug needed for a therapeutic effect tends to be so high that you end up needing to take in grams to kilograms of it to achieve those effects, which comes with obvious toxicity risks that make use of the drug unacceptable.
Related metrics exist under a similar formalism. For example, the CC50 (cytotoxic concentration) describes the concentration concentration needed to kill half of the cells in a culture. Ideally, there is a huge gap between the CC50 and the IC50 for the drug you’re looking at. Additionally, some drugs enhance the activity of enzymes, in which case their effectiveness can be reported as an EC50 (effective concentration) value which is the concentration of drug needed to achieve the half-maximal effect. Antibodies’ ability to neutralize is often reported as an NT50 (neutralizing titer), the titer required to reduce infection of cells by a virus (or exert toxic effects on them in the case of a toxin) by 50%.
Ensitrelvir is interesting- in RCTs it showed a signal of reducing the risk of neurological long COVID symptoms in a study that allowed vaccinated participants based on data presented at CROI and unlike nirmatrelvir, it does not require ritonavir to boost its levels. It also has a different binding site on MPro than does nirmatrelvir, meaning different mutations are needed to escape it relative to nirmatrelvir. However, despite not needing ritonavir, it still seems to have a number of concerning drug-drug interactions. More data are needed to understand the value of this antiviral.
eGFR is a measure of kidney function basically describing how much blood the kidney is able to process over a period of time, generally milliliters per minute. Higher eGFRs reflect superior kidney function.
Some have proposed that use of Paxlovid in those with this degree of renal impairment may be done safely but there are no clinical guidelines from relevant expert bodies that suggest giving Paxlovid to these patients.
Resistance mutations to nirmatrelvir are well-documented and have existed since even before Paxlovid had gained use. These typically come at a massive fitness cost to SARS-CoV-2, although recent evidence suggests that there are pathways by which resistance can be acquired without this substantial cost. Nevertheless, resistance is not widespread enough at this point to be a serious concern for taking or prescribing Paxlovid.
I find VV116’s existence a bit funny because it is so similar structurally to remdesivir but they replaced one of the hydrogens with deuterium. In one of their papers they describe how this deuterium is really important for the pharmacokinetics but in reality… it’s probably just a way around Gilead’s patent on remdesivir. Also while writing this I learned that VV116 is apparently authorized for use in Uzbekistan, which is neat.
I don’t think that self-reported data are a huge problem here given the squishiness of long COVID as a diagnosis and a lack of objective criteria for diagnosis, but it does speak to a major methodological problem with long COVID work.
Others do exist and are in the pipeline but the only ones used clinically before were the amantadines (M2 inhibitors) which are no longer used for influenza because of widespread viral resistance. Nonetheless, there are next-generation M2 inhibitors in the pipeline which should (in theory) be effective against current circulating viruses.
Resistance to NAIs is interesting. Obviously, neuraminidase can evolve to fail to bind the NAI, but in addition to this, mutations in hemagglutinin that reduce the affinity of the protein to sialic acid also confer resistance (as this helps prevent the virus from getting stuck when it tries to leave the cell). Some data suggests that isolates of influenza that are resistant to NAIs tend to have less ability to transmit, offering hints as to why NAI resistance might be so relatively rare.







