That "post-vaccine syndrome" preprint everyone is talking about
There are major issues with the conclusions being drawn from it.
This past week, there has been a bit of a firestorm over social media over this preprint, with claims like “this proves that long COVID was a vaccine injury all along,” or “Aha! I told you those vaccines were unsafe!" when it reality it shows nothing like that. Truthfully, the preprint has major issues which prevent any meaningful conclusions from being drawn, and has had a major ethical problem which (as of the time of writing this) still remains unaddressed has been recently corrected. Let’s look at this preprint together.
The study the preprint described recruited 42 people who were described as experiencing a post-vaccine syndrome (PVS) and compared them to 22 healthy controls. The breakdown of vaccines received by the PVS cohort based on medical records available for 39 of them is:
Comirnaty (Pfizer) (n=14)
Spikevax (Moderna) (n=21)
Jcovden (J&J) (n=4)
PVS participants reported the following symptoms:
excessive fatigue (85%)
tingling and numbness (80%)
exercise intolerance (80%)
brain fog (77.5%)
difficulty concentrating or focusing (72.5%)
trouble falling or staying asleep (70%)
neuropathy (70%)
muscle aches (70%)
anxiety (65%)
tinnitus (60%)
burning sensations (57.5%).
In other words, a lot of nonspecific symptoms which are similar to those reported by people experiencing long COVID and ME/CFS. Of those 42 who reported experiencing PVS, 15 (35.7%) and 10 (45.5%) of the controls reported a SARS-CoV-2 infection at least once.
Right off the bat, we have some major problems here.
Firstly, while all of these symptoms can be and often are debilitating, they are very nonspecific. It is hard to claim that these symptoms can identify an individual as having PVS. Furthermore, we don’t actually have any rigorous definition of PVS here. In fact, if we’re to get technical, this work doesn’t even substantiate that there exists a unique clinical entity known as PVS (though more on that later). This is not me saying that PVS does not or cannot exist (certainly, in very rare cases, people can experience serious adverse events after vaccination, which, potentially can become chronic), but just by the setup of the study so far, we are expected to take it on faith both that PVS exists and this cohort is representative of members of the condition, but this work does not provide sufficient evidence for that. Another criticism raised about this preprint is that the sample size used here is small- this is undeniably true. However, if you’re looking at a very rare condition (as PVS would certainly have to be given the degree of our pharmacovigilance for the COVID-19 vaccines which managed to identify adverse events occurring on the order of 1 in 1,000,000 to 1 in 100,000 people), a sample size like this may be the best you can do. However, it does result in a number of statistical issues (which I will come back to).
One major challenge with claiming that these patients’ symptoms were associated with the vaccine is that a bunch of these patients (35.7%) did have evidence of a prior SARS-CoV-2 infection based on nucleocapsid antibodies (nucleocapsid is not in the vaccines used in this study, so antibodies can only result from infection). The decision to check for nucleocapsid antibodies is an important step for improving the rigor of the study to help address whether or not the patients’ disease might be from COVID-19 rather than a vaccination, but there are 2 significant limitations here. The first is that vaccinated people are less likely to generate antibodies to N upon infection because they have more robust and rapid control of the infection (a fact that showed us that the Moderna vaccine was preventing asymptomatic infections as well as symptomatic ones in its prelicensure trial). The second is that N antibodies can absolutely form after infection, but they can wane to undetectable levels over time:
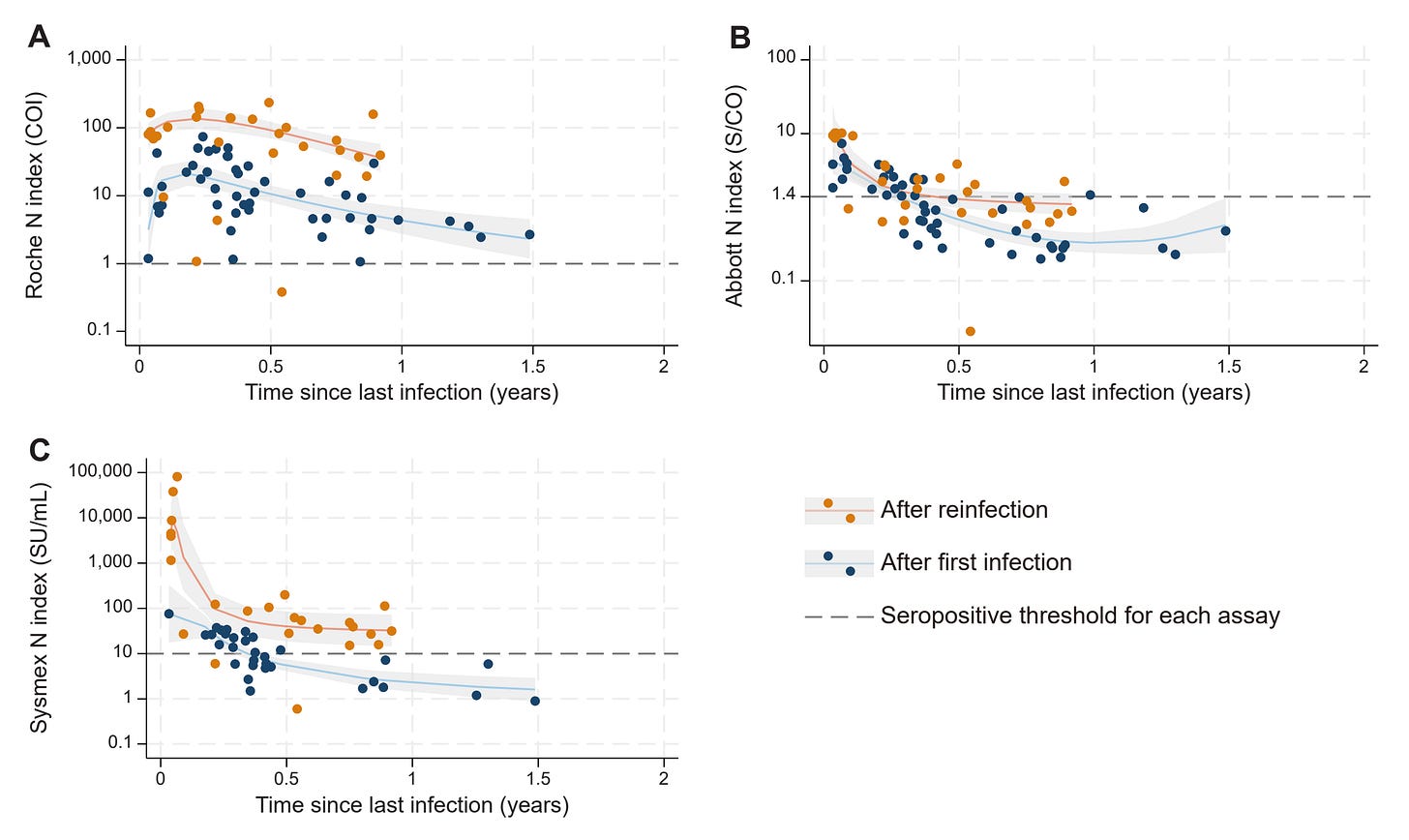
In the above study, nucleocapsid antibodies could fade to undetectable levels in fewer than 6 months- and participants had a blood draw anywhere from ~4 months to ~2 years since their last vaccine dose:
It should also be noted that patients with ME/CFS-like disease might not be as effective in generating or retaining antibody responses relative to those who do not have these conditions, which may potentially extend to nucleocapsid antibodies. This makes it even harder to say that these patients’ symptoms did not result from a SARS-CoV-2 infection that they might not have been aware of and thus ultimately reflects PASC. Still, let’s pardon this for now and look at the study results.
The major claims made by the study are (in no particular order):
PVS patients had more reactivation of Epstein-Barr virus (EBV)
Exhausted CD4 and CD8 T cells more frequent in the PVS patients
increased TNFα+ CD8 T cells in PVS patients
PVS patients had higher levels of circulating spike protein than controls
Distinct autoantibody profiles in the PVS patients
Let’s go down the list.
EBV Reactivation
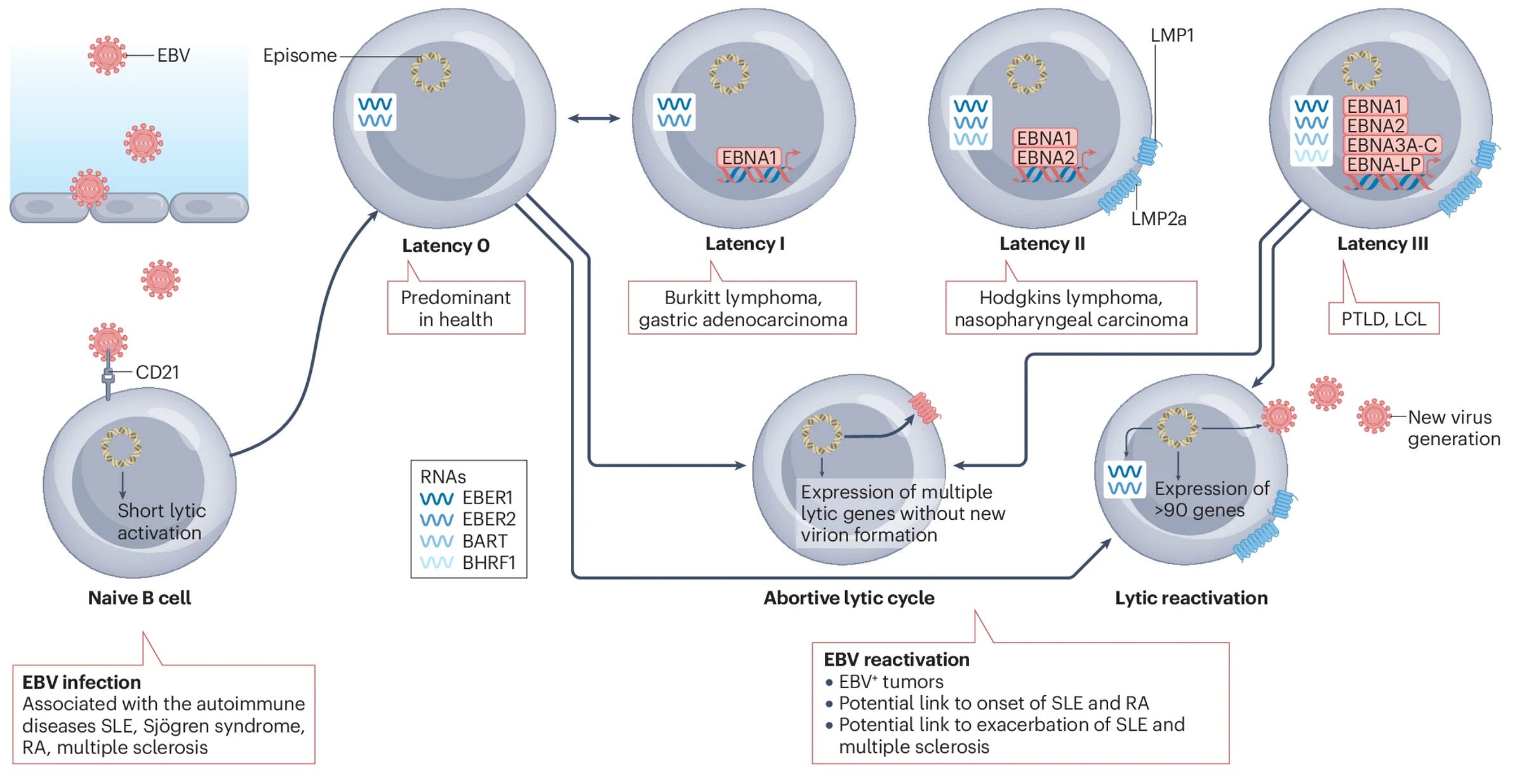
Epstein-Barr virus (EBV) is a herpesvirus (specifically, a gammaherpesvirus) also known as human herpesvirus-4, which is responsible for a broad range of conditions including infectious mononucleosis, multiple sclerosis, diffuse large B cell lymphoma (DLBCL), Burkitt’s lymphoma, NK/T cell lymphoma (NKTL), HIV-associated lymphomas, post-transplant lymphoproliferative disorder, Hodgkin’s lymphoma, gastric carcinoma, oral hairy leukoplakia, and nasopharyngeal carcinoma. Once EBV infects a person, it can persist in a latent form which does not express antigens until a suitable cue occurs for it to reactivate (the major cell that enables latency is the B cell and it is thought that activation of the B cell receptor can cue EBV into re-establishing a lytic replication cycle). Because of this, EBV can reactivate throughout the lifetime, sometimes causing symptoms or leading to major disease.
Detecting EBV reactivation can be done in any of several ways, but the team in this preprint used serology only (i.e., looking at antibodies to EBV lytic antigens in the blood). The problem here is that all measurements in this study are based on a single blood sample taken once for each participant. Without a prior sample to compare it to, you cannot conclude that EBV reactivation was more frequent in the PVS group than in the control group because it remains possible that the PVS group just has higher reactivity to EBV at baseline. I have trouble understanding why they would not do a qPCR to look at the number of EBV genomes expressed in the blood or saliva of each participant, as this would be a direct readout of the burden of EBV in each participant.
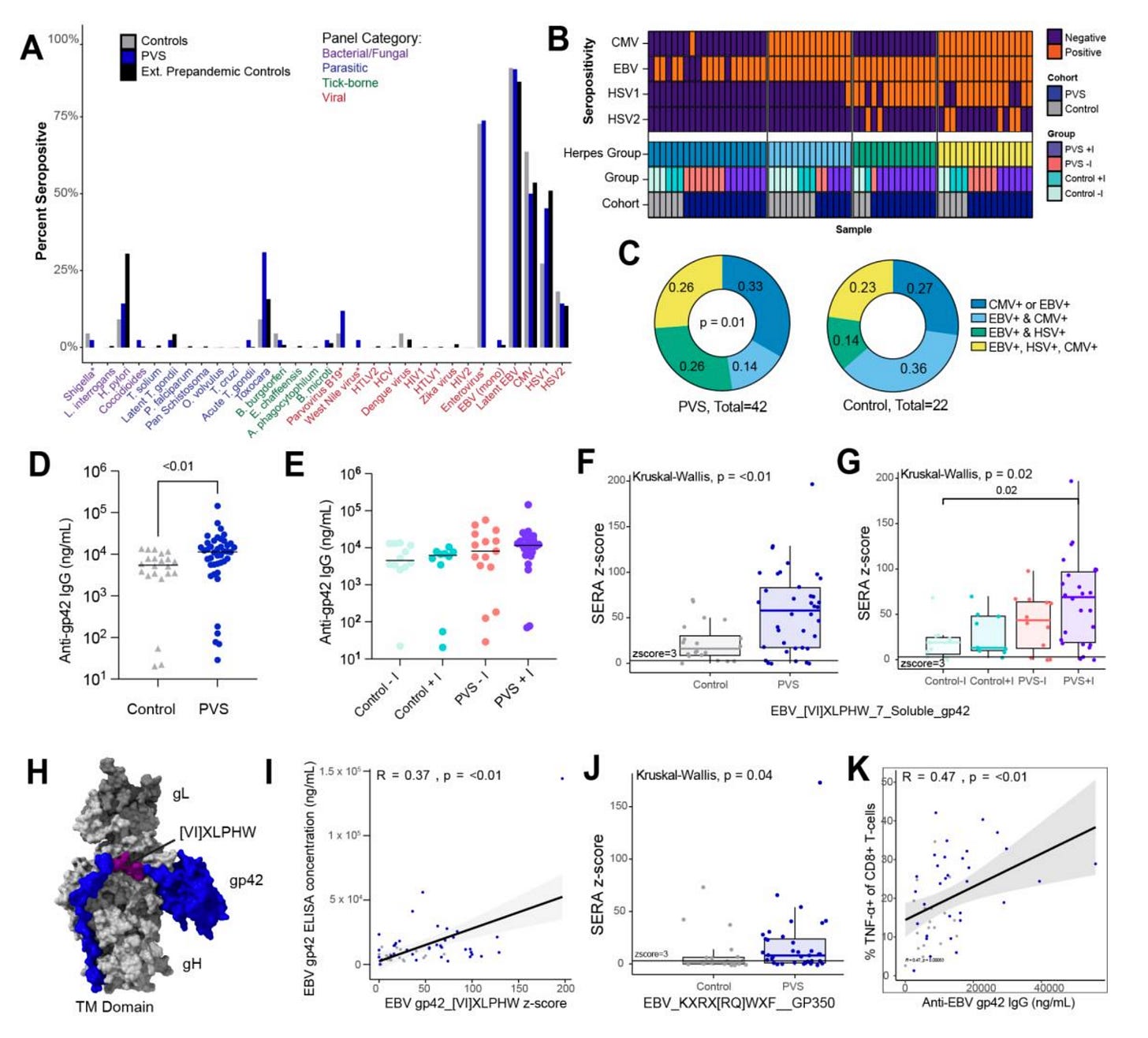
Still, the methodological qualms aside, the differences observed in reactivity to EBV gp42 are minimal and driven by a few outliers in the PVS group. The team did find antibody responses to two specific regions to be enriched in the PVS group (gp42 peptide [VI]XLPHW and gp350 peptide KXRX[RQ]WXF), which may be meaningful. In figure 4K, the team does note a trend of increasing TNFα+ CD8 T cells with higher antibody reactivity to gp42, which may potentially reflect more recent reactivation, but again, cannot be definitive without at least an earlier blood sample to compare it with.
T cell exhaustion
At this point, this is a term that makes my eyes glaze over because of how often it is misused, but it’s worth explaining. When the immune system responds to threats, this results in damage to healthy tissue as well as infected tissue. This could be particularly problematic if you have a chronic infection or cancer, because this would mean continuous damage to healthy tissues in response to that threat. As an adaptation to this, T cells can become “exhausted,” meaning that, though they still respond, they do so less robustly in the setting of chronic infection and in cancer (a lot of cancer immunotherapy revolves around blocking the signaling that produces T cell exhaustion). Exhaustion can be a bit of a double-edged sword. By having less function, you reduce tissue damage by the T cells, but you might also forfeit more effective control of the chronic infection or tumor through the immune response.
Here’s the tricky part: there is no simple way to look at a T cell and conclude that it is exhausted. We know that exhausted T cells tend to have more co-inhibitory molecules on their surface (e.g., PD-1, CTLA-4, TIM3, LAG3, TIGIT, etc.) but the trouble is that surface markers alone won’t tell you that a T cell is exhausted because the thing that induces all of these markers is… activation of the T cell receptor (TCR). In other words, cells with very high levels of “exhaustion markers” can still be highly active, functional cells- as was shown with T cells from COVID-19 patients. Anyway, with that preamble, let’s look at the data.
For 2D, there are statistically significant differences reported for the levels for CD4 Tems (effector memory), rnTreg CD4s (resting natural regulatory CD4 T cells) and CXCR3+ CD4 T cells. There’s a bit of an issue here though. Firstly, in addition to looking at statistical significance, it is worth considering the spread of the data in each group. Ideally, you would like for there to be a clear difference in the distributions i.e., given some value for the percent of CD4 T cells, you should ideally be able to predict whether it came from the control or the PVS groups. I think that is exceptionally difficult to do for essentially all of the distributions where a statistically significant difference is noted. In 2E, the T cells were stimulated and observed for their cytokine response. There is a difference in IL-4+ CD4 T cells and IL-4/IL-6+ T cells at the statistical level, but the difference is entirely driven by a handful of outliers.
Moving ahead, Figure 2F notes there are more CD8 Tcms (central memory T cells) in the blood, more CD8 Tex (exhausted T cells), and more TNFα+ CD8 T cells. Because there was no functional assay here and this was based solely on surface markers, I am not able to accept that there is bona fide CD8 T cell exhaustion here as much as CD8 T cells expressing high levels of biomarkers that might (but might not) indicate exhaustion. Increased levels of central memory T cells (Tcms) alone is hard to interpret as far as the clinical importance, but may reflect a heightened state of inflammation. A similar interpretation I think follows from the finding of increased TNFα+ CD8 T cells in the PVS group- something is causing a persistent inflammatory response in the body.
One point I am troubled by in this preprint is that all measurements are expressed as percentages, without absolute counts of the cells being offered. This would be valuable information that is needlessly forfeit. As a minor point- I don’t really understand the choice to represent the data here in violin plots (as opposed to beeswarm plots) because it forces you to include a y-axis that has negative values, which is impossible to have for these types of experiments.
Spike in the blood
This is the part of the story that I think has gotten the most attention and is, I think, the messiest. I previously discussed the challenge of determining whether or not the PVS group actually reflects long COVID cases- and the data on the spike protein in the blood doesn’t help with answering this.
First, we should take a moment to look at Figures 5B and 5C. It’s key to remember that there was only a single blood draw here, so we can’t claim things like “there is an increasing level of spike protein over time,” as these are all different participants. It is also implausible for the source of the spike protein to be mRNA vaccines if the last dose was over 2 years ago. A positive spike protein is reported on their immunoassay as late as 709 days after the last vaccine dose. However, in terms of the levels, though there are more individuals in the PVS group (whether or not they reported an infection) who have detectable spike, the difference in their levels is driven by a few outliers. That said, why would there be spike 709 days after the last vaccine dose in someone who never reported a SARS-CoV-2 infection?
The simplest answer is: there was a SARS-CoV-2 infection and they didn’t know about it or didn’t report it (which is I think the most likely reason). The other possibilities require an understanding of the immunoassays used to detect spike protein. These used a technique called SPEAR which is explained by the company SpearBio in the video below:
The basic idea isn’t particularly complicated- you use 2 antibodies that bind to a different part of the antigen of interest (in this case, spike) and they are linked to a DNA probe. You then add a DNA polymerase enzyme, which can extend the probe when the two probes are linked close enough together. If two extensions occur, it means that both antibodies bound to the antigen in question. Trouble is: there are some important things that could give you a false positive antibody result.
One is anti-idiotypic antibodies:
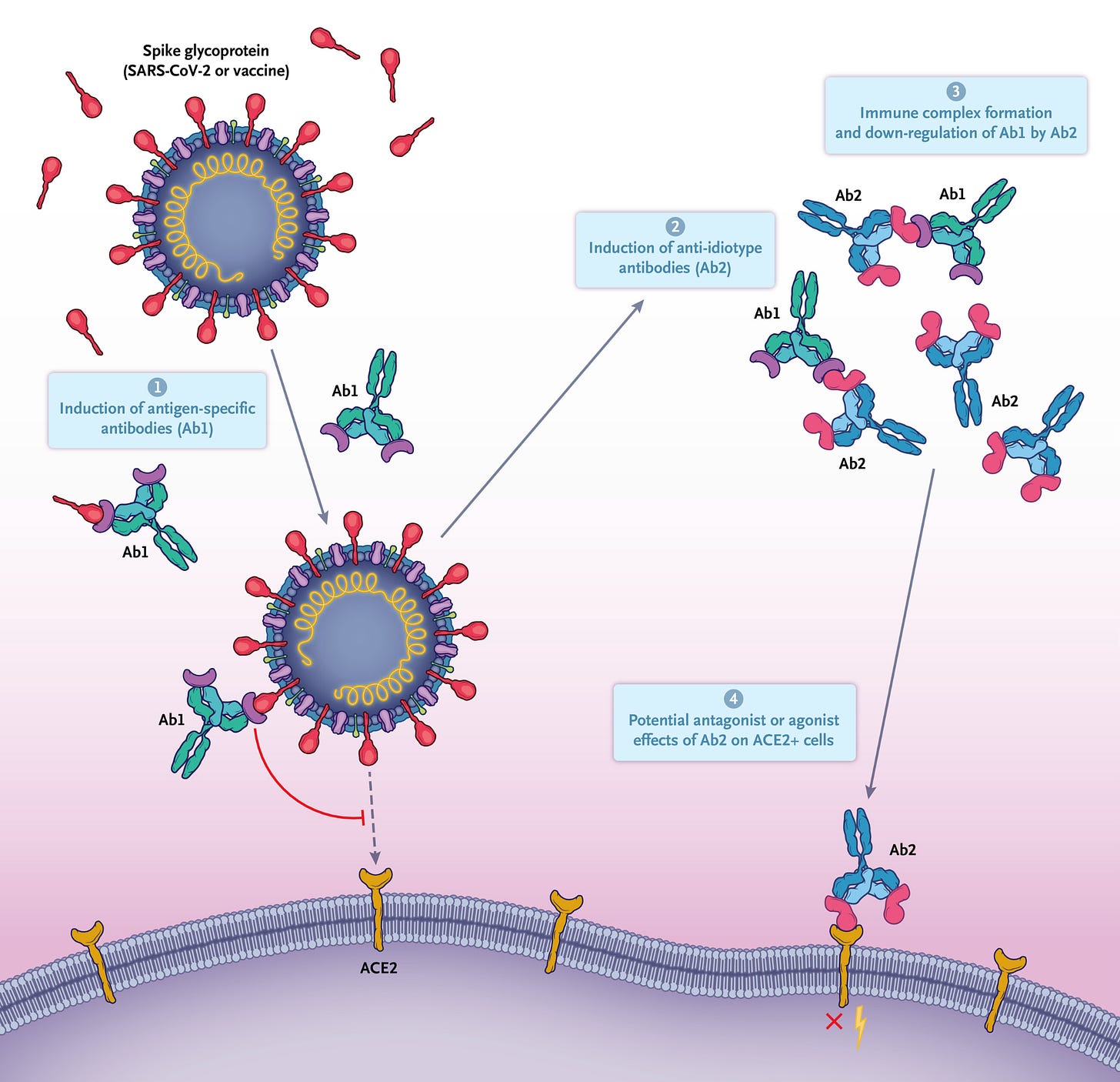
Basically, in the course of an immune response, you make antibodies against a target, but, sometimes you may make antibodies against those antibodies, which can mimic the original target. So, for example, if responding to the spike protein, you might make an antibody whose binding surface resembles ACE2 (the SARS-CoV-2 receptor). If you then make an antibody to the ACE2-like region of that antibody, you can have an antibody that mimics spike protein. Thus, the assay could appear to contain spike protein when in fact it is an anti-idiotypic antibody. Still, the team did use both 2 S1 antibodies and antibody recognizing S1 and another binding S2 as a validity check. This makes it less likely (though not impossible) to get a positive signal.
The other big thing that could cause a false positive signal is: rheumatoid factor. Rheumatoid factor refers to antibodies (usually IgM) that recognize the constant regions of antibodies:
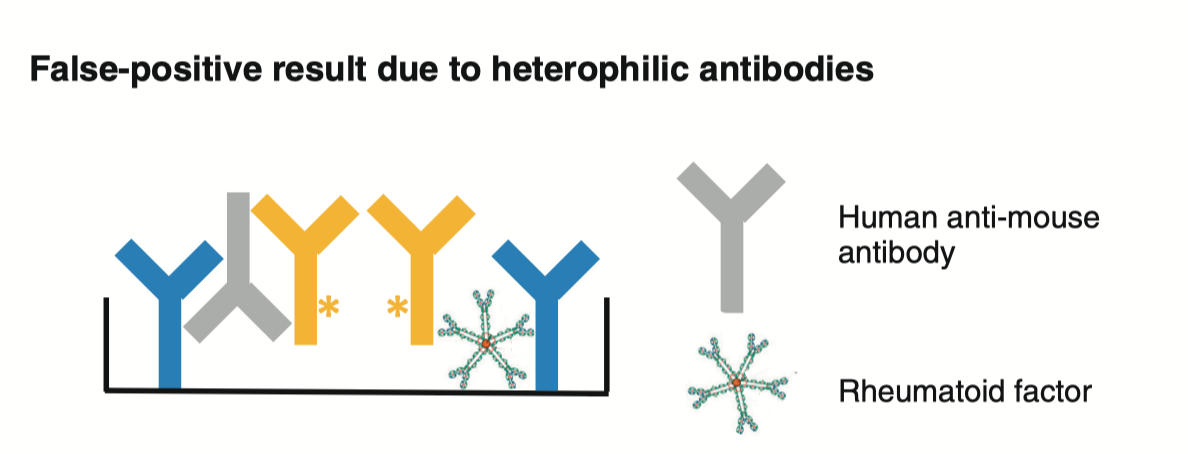
In this assay, signals occur when the two probes on the two different antibodies are in close proximity to one another- which the presence of rheumatoid factor could force. The team did test for a number of autoantibodies, but as far as I could tell, did not check for rheumatoid factor. This potential confounder could have been avoided by purifying out the antibodies from the blood before performing the assay.
Autoantibodies
The heat map above shows the distribution of autoantibodies and the different class of antibody they fall into (IgM, IgG, and IgA) and there are significant differences between the PVS cohorts and the controls. AQP4 autoantibodies (IgG) seem to be enriched among the PVS group. These antibodies are known for causing neuromyelitis optica spectrum disorder (NMOSD). The other 2 that stood out were IgG against histone H1, which were much less common in the PVS cohort, and IgM against nucleosome, which were much more common in PVS than in controls. Nucleosome autoantibodies may be an indicator of systemic lupus erythematosus (aka lupus) activity.
Undisclosed COIs
Update: as of February 26 2025, the COI section has been updated:
By far my biggest issue with the preprint is that two of the others have MAJOR conflicts of interest which are not disclosed:
Danice Hertz is on the advisory board of react19, which is organizing class action lawsuits over vaccine injuries from COVID-19 vaccines.
Brianne Dressen is an anti-vaccine activist who has published a book about her purported vaccine injury, and filed multiple lawsuits through her “vaccine injury group” react19, and is suing Astra Zeneca.
On their own, these are not disqualifying factors in helping to conduct a study, but the failure to disclose such major conflicts of interest is one of the most severe breaches of ethics that can occur with a researcher (being charitable with the title). This is in fact what Andrew Wakefield did with his MMR fraud (among a number of other profoundly unethical acts). Beyond the mere breach on the part of the researchers, it is unacceptable that the lead authors would not ensure that these conflicts of interest were disclosed, be it from a lack of vetting on their part or a failure to enforce it.
Conclusions
It is honestly hard to draw any conclusions from this work directly. The fact that only a single blood draw is taken from these patients and at significantly more time after their vaccination than would be ideal greatly constrains any claims that can be made. I can agree that there is a distinct autoantibody profile in the PVS cohort than in controls, but the meaning therein is not immediately obvious. There are minimal, if any, differences observed in terms of the proportions and distributions of the examined immune cells. The study is hamstrung by its limited sample size because this greatly reduces its statistical power. That said, given that this condition would have to be extremely rare (if it exists at all), this may not be an unreasonable sample size- but it is one that makes it impossible to make sound conclusions about PVS. The preprint also does not at any point substantiate PVS as a unique syndrome. It would be ideal if there were prospective blood samples taken from people on regular intervals following vaccination which could be assessed and then a subset that developed symptoms consistent with PVS could be compared with matched controls.
What could have been done better for next time?
Scientists do not have the right to insulate themselves from the broader context of the society they live in, of which we are a part. At this moment, all of our scientific institutions are being razed on the whim of an unelected billionaire and we have an HHS Secretary who has been prattling for decades about harms of vaccines that categorically do not exist and has even claimed that there is no such thing as a safe and effective vaccine. He has managed to postpone a meeting of the Advisory Committee on Immunization Practices indefinitely which included agenda items like determining whether particular vaccines should be covered under the Vaccines for Children (VFC) program, and is reportedly in the process of replacing ACIP members with his sycophants based on perceived conflicts of interest (a post for the immediate future). It is extraordinarily disappointing that serious scientists with excellent reputations would time the publication of this preprint at this moment. Rather, it was not just timed for this moment, but there were press releases and a New York Times article published about it. There were claims that the authors were unaware that it would have such an impact among anti-vaccine lobbyists. I don’t find that plausible. Still, this discussion would be incomplete without noting that there is a competing ethical obligation to publish. The sympathetic part of me acknowledges that these patients have been suffering profoundly without an explanation or effective treatment for a very long time, and, whatever the basis of their condition, that should not be the case. The argument therefore could be made that there is a moral obligation to share work in this field as soon as it is completed. Except… this work doesn’t actually advance the field in any meaningful way. Though exploratory, I see no obvious leads for targeted therapy. There are no reliable conclusions that can be made from the work because of the limitations of the methods and the choice to use only a single blood sample at uncontrolled time periods from vaccination. If this work had a chance of meaningfully advancing the knowledge in ME/CFS and related syndromes, there could be a case that it should be published as soon as possible. But it doesn’t. People suffering from ME/CFS and related conditions deserve high-quality science to understand the underlying nature of their conditions and trial therapies in a rational way. This doesn’t do that. So, as far as what specifically could be done for next time:
Publish when your data is meaningful- when it has a chance of leading to something to improve the care of patients with this condition or point out that a current line of therapy is inappropriate.
Be aware of the context in which you live and understand how your work will be used to fuel the agenda of anyone who finds it suitable. Do not make it easy to do this. If there are obvious bad faith interpretations of the work (as there were here) refute them explicitly within the work.
Consider how much publicity your findings actually deserve.












Always learn from your work Ed. Would love to see a post or 3 on what is fairly settled science on post viral syndromes . You touched on some of that history in this post when you mentioned all the potential effects of EBV . As a primary care doc of almost 40 years , it makes me think of so many syndromes , complaints , peculiar symptoms that I could just barely explain to at least walk through the problem with the patient. I many times suspected a post infectious cause but had almost no way to confirm that. As a first step to help these folks , I would love to do more to let them know this is why you are suffering . Thanks.