[Imported Post] Vaccine Shedding Doesn’t Work Like That
This post goes through what shedding is, which vaccines can shed, and what that means for people's health.
I am working on consolidating all of my posts on one site (substack) but unfortunately I have to do this manually as substack does not want to let me import my own materials automatically. This post reflects information current as of May 17, 2021. However, there are still no live-attenuated or otherwise replication-competent COVID-19 vaccines available to the public as of February 27, 2024.
The short version: COVID-19 vaccines are not even theoretically capable of shedding. Attributing health effects to contact with a vaccinated person because of their COVID-19 vaccine is completely baseless and anyone who is experiencing health problems should seek appropriate care from a qualified medical professional rather than dismissing it as the result of “shedding spike protein.”
People claiming that vaccinated people are shedding the spike protein which is leading to all sorts of terrible things, which is not how anything works. Let’s talk about what vaccine shedding actually is and how it works.
"Shedding" refers to the ability of an individual to release infectious particles into the environment, potentially passing them to others (horizontal transmission). This does NOT mean that those who encounter the shed particles become sickened. For one thing, we’re all very familiar at this point with asymptomatic infections. For another, the vaccines which are theoretically capable of this use such weakened forms of the pathogens in question that becoming sick is a true rarity (with the exceptions I will shortly discuss).

Vaccine shedding is only theoretically possible with live-attenuated vaccines (LAV). These are vaccines that contain an infectious organism (a virus or bacteria) which can replicate to a limited extent within a vaccinated person (vaccinee). There are a few ways to make them, but the general idea is that you take a culture of the pathogen and grow it for many generations in conditions that don’t resemble those in the body, causing it to adapt to them and be unable to cause disease in humans. For example, the measles vaccine strain (Edmonston B) was made by growing it for 24 generations in kidney cells at 35°C to 36°C , then in chicken embryo cells for 28 generations, and then in chick embryos for another 6 generations, and then once more in chick embryo cells. From this several other strains of measles have been derived (including the further attenuated Moraten strain used in US vaccines). Because these conditions lack the selection pressure of a host’s immune system, the virus gradually loses virulence because it doesn’t need it and maintaining it is evolutionarily expensive. These vaccines cannot cause disease in the vaccinee unless the vaccinee is severely immunocompromised and the immunodeficiencies that would be relevant for this are screened for at birth with the heel prick test. LAVs include MMR-II (measles, mumps rubella) vaccine, childhood varicella vaccines (Varivax and MMRV), nasal flu vaccine (FluMist), Rotavirus (Rotateq, Rotarix), and the oral polio vaccine (OPV- not used in the US).
While the presence of a functional, replicating infectious agent is necessary for shedding to occur, it is not sufficient. There has never been a single well-documented (note this qualifier) case of a vaccinee horizontally transmitting vaccine-strain measles, mumps, or rubella to another individual. Rubella in MMR can persist in sera for 7 to 28 days after vaccination but at levels below what are needed to initiate a productive infection and thus cannot horizontally transmit from vaccinees. Shedding has been observed very rarely for some strains of mumps but not the Jeryl Lynn strain used in the US vaccines. Of about 50 million doses of chickenpox (varicella) vaccine given, there have been 11 documented cases of transmission from the vaccinee; this requires direct contact with the vaccinee's rash and not all vaccinees develop a rash. If I had to venture a guess as to why horizontal transmission isn’t observed with these vaccines, it would be that the viruses do not have an opportunity to replicate to infectious levels within the respiratory tract as they are passed through the inhalation route but I cannot say definitively.
Nasal flu vaccines contain live-attenuated influenza virus (LAIV); while shedding has been noted before, there has never been a documented case of influenza occurring from horizontal transmission of vaccine-strain virus to an immunologically competent person. Vaccinated children shed sub-infectious quantities of the virus for several days. This does not occur with injected flu vaccines (as they are inactivated vaccines that contain no replication-competent particles).
Shedding is known to occur with rotavirus vaccines. This occurs without symptoms; 93% of vaccinees in one study were found to have rotavirus in their stool for 5-10 days. This is considered a good thing because it reflects that the virus is replicating within the GI tract of vaccinees and this is thought to be critical for protective immune responses. Symptomatic gastroenteritis has been documented because of horizontal transmission with this vaccine, but this is exceptionally rare (this was the only case of it I could find and if you read it, some fairly unusual things happened; reviews on the subject note that there is no documented increased risk of gastroenteritis among contacts of the vaccinee in the risk period meaning the risk is too small to be accurately measured) and in general shedding does not result in any symptoms (the rotavirus vaccine generally doesn’t even cause gastroenteritis in the vaccinee). The major takeaway here is to observe care when changing the diapers of recently rotavirus vaccinees (which really should be a rule regardless of whether or not your child has recently gotten their rotavirus vaccine).
The oral polio vaccine is used for polio eradication campaigns because the inactivated vaccine (IPV, which the US uses) does not induce good mucosal immunity in the GI tract. As a result, in endemic areas, vaccinees can still become infected and transmit polio (though they don't get sick) and so, IPV is insufficient. In areas where vaccine uptake is inadequate, OPV from the vaccine can circulate among the population to a significant extent, which does grant immunity but with a caveat. This can result in a reversion to neurovirulence where vaccine-strain poliovirus can cause polio (vaccine-associated paralytic poliomyelitis, VAPP). VAPP is very rare- 4.7 cases are estimated per million births, but it poses a real problem for polio eradication. Most higher income nations do not use OPV largely because of the risk of VAPP. There is a next-generation oral polio vaccine, nOPV2, which has additional mutations engineered into it that prevent reversion to neurovirulence and is gradually being introduced for polio eradication. This is basically the only case where the transmission of a vaccine is clinically important.
Now we get to the COVID-19 vaccines. At this point we have successfully concluded that a vaccine must contain replication-competent pathogens to be able to shed, and that even if it does, this is typically not sufficient to cause disease. The conspiracies running rampant about the effects of shedding spike protein therefore make no sense as none of the vaccines available currently for COVID-19 are live-attenuated. mRNA vaccines (Pfizer and Moderna) lack any machinery to replicate. What about Johnson and Johnson/Janssen? They use a replication-incompetent adenovirus vector. The vector has its E1 gene deleted which prevents the virus from initiating a replication cycle. It is not a requirement that vectored vaccines be replication-incompetent. The use of a nonpathogenic or minimally pathogenic vector is appropriate for certain infectious diseases, especially those where a reversion to virulence would be especially serious (such as Ebola). There are several Ebola vaccines. One of these uses a recombinant vesicular stomatitis virus (rVSV) vector which expresses ebola envelope glycoprotein on its surface- it has demonstrated efficacy as high as 100% in clinical trials. VSV does not cause disease in humans, but it can cause it in livestock. There was therefore concern about VSV-vectored vaccines being shed from vaccinees and causing disease in livestock, but a recent study suggests this does not occur at the doses at which the vaccine is ordinarily given. Clinical trial experience with this vaccine showed shedding to be an infrequent occurrence. Shedding studies are not required for replication-incompetent vectors. Therefore it has no capacity to shed either.
So this nonsense about vaccinees shedding spike protein really should be disregarded with prejudice and if you are experiencing health concerns, please do not presume them to be the result of contact with a vaccinated person and seek professional medical care to address the issue.
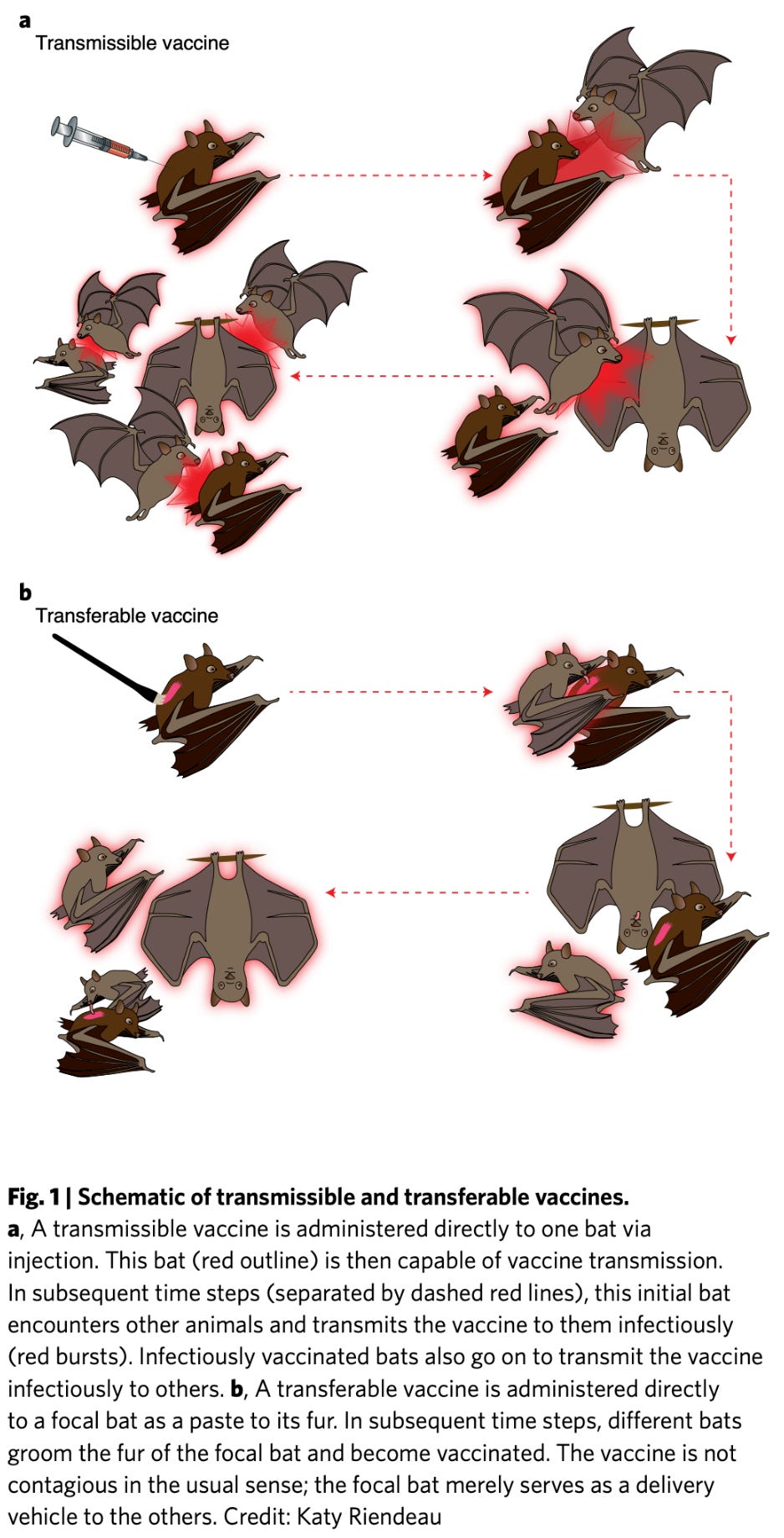
Now, all this notwithstanding, there is actually a strong case to be made for transmissible vaccines. For instance, zoonotic diseases (those that come from animals and become established in humans) are a serious challenge for eradication campaigns because the disease can keep getting reintroduced into the population via an animal reservoir. Realistically, we cannot vaccinate every single animal that may act as a source for the infection. But what if we could vaccinate just one, or even a few animals and have them pass the vaccine to each other, developing immunity? Suddenly a vaccine that horizontally transmits sounds like quite a good idea, doesn’t it? Nuismer and Bull explore the concept in detail in their publication. Of course, polio in particular shows us that there can be issues when you extend this to humans. Severely immunocompromised individuals can be harmed by live attenuated vaccines, and if a vaccine spreads throughout the population in this manner, this can become an issue. Here though, polio seems to also inspire the solution: engineer additional means to prevent a reversion to virulence. Alternatively, you could use a non-pathogenic vector (although this isn’t a magic bullet; rVSV is a brilliant technology but it can clearly fail as shown with Merck’s candidate COVID-19 vaccine). The concept of a transmissible human vaccine isn’t quite ready for prime time yet, but consider how valuable this would be in responding to pandemics (and we will most assuredly have more and I fear they might make COVID-19 look cute by comparison- but that’s another post unto itself). I think it’s worth pursuing further (along with self-boosting vaccines).
In summary:
The construction of a vaccine that can readily horizontally transmit from vaccinees is a worthwhile goal for preventing outbreaks of disease, particularly zoonoses. However, it has not yet been achieved.
The shedding of vaccine-strain viruses from several vaccines is of minimal clinical consequence and is essentially only relevant for those who are severely immunocompromised. It is also exceptionally rare and largely limited to the rotavirus and oral polio vaccines.
None of the COVID-19 vaccines currently available are capable of shedding because none of them are live attenuated. Therefore any claims about health consequences due to COVID-19 vaccine shedding from vaccinees are readily dismissible as fictional.
References
Bull JJ, Smithson MW, Nuismer SL. Transmissible viral vaccines. Trends Microbiol 2018;26(1):6–15.
Greenwood KP, Hafiz R, Ware RS, Lambert SB. A systematic review of human-to-human transmission of measles vaccine virus. Vaccine 2016;34(23):2531–6.
Nuismer SL, Bull JJ. Self-disseminating vaccines to suppress zoonoses. Nat Ecol Evol 2020;4(9):1168–73.
Bennett A, Pollock L, Bar-Zeev N, et al. Community transmission of rotavirus infection in a vaccinated population in Blantyre, Malawi: a prospective household cohort study. Lancet Infect Dis [Internet] 2020;Available from: http://dx.doi.org/10.1016/S1473-3099(20)30597-1
Tsolia M, Gershon AA, Steiberg SP, Gelb L. Live attenuated varicella vaccine: Evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. J Pediatr 1990;116(2):184–9.
Kulkarni PS, Jadhav SS, Dhere RM. Horizontal transmission of live vaccines. Hum Vaccin Immunother 2013;9(1):197.
Bennett A, Pollock L, Jere KC, et al. Infrequent transmission of monovalent human Rotavirus vaccine virus to household contacts of vaccinated infants in Malawi. J Infect Dis 2019;219(11):1730–4.
Altered Immunocompetence [Internet]. Cdc.gov. 2021 [cited 2021 Apr 20];Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html
Custers J, Kim D, Leyssen M, et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine [Internet] 2020;Available from: http://dx.doi.org/10.1016/j.vaccine.2020.09.018
ICH Considerations General Principles to Address Virus and Vector Shedding [Internet]. Europa.eu. [cited 2021 Apr 20];Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-10.pdf
Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020;5(1):91.
Newborn screening information for severe combined immunodeficiency [Internet]. Babysfirsttest.org. [cited 2021 Apr 20];Available from: https://www.babysfirsttest.org/newborn-screening/conditions/severe-combined-immunodeficiency-scid
U.S. Vaccine Names [Internet]. Cdc.gov. 2020 [cited 2021 Apr 20];Available from: https://www.cdc.gov/vaccines/terms/usvaccines.html
vaccine-shedding - The Immunization Partnership [Internet]. Astoundz.com. [cited 2021 Apr 20];Available from: http://immunize.astoundz.com/blog/2016/september/24/vaccine-shedding/
Markkula J, Hemming-Harlo M, Vesikari T. Shedding of oral pentavalent bovine-human reassortant rotavirus vaccine indicates high uptake rate of vaccine and prominence of G-type G1. Vaccine 2020;38(6):1378–83.
OPV Cessation [Internet]. Polioeradication.org. [cited 2021 Apr 20];Available from: https://polioeradication.org/polio-today/preparing-for-a-polio-free-world/opv-cessation/
Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014;210 Suppl 1(suppl 1):S380-9.
Alexander JP, Ehresmann K, Seward J, et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 2009;199(3):391–7
Nasal Flu Vaccine [Internet]. Ox.ac.uk. [cited 2021 Apr 20];Available from: https://vk.ovg.ox.ac.uk/vk/nasal-flu-vaccine
Tell JG, Coller B-AG, Dubey SA, et al. Environmental risk assessment for rVSVΔG-ZEBOV-GP, a genetically modified live vaccine for Ebola virus disease. Vaccines (Basel) 2020;8(4):779.
Morozov I, Monath TP, Meekins DA, et al. High dose of vesicular stomatitis virus-vectored Ebola virus vaccine causes vesicular disease in swine without horizontal transmission. Emerg Microbes Infect 2021;10(1):651–63.
Annex 2 Guidelines on the quality, safety and efficacy of Ebola vaccines [Internet]. Who.int. [cited 2021 Apr 20];Available from: https://www.who.int/biologicals/areas/vaccines/Annex_2_WHO_TRS_1011_web-2.pdf?ua=1
Vrba SM, Kirk NM, Brisse ME, Liang Y, Ly H. Development and applications of viral vectored vaccines to combat zoonotic and emerging public health threats. Vaccines (Basel) 2020;8(4):680.
Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination - response from the manufacturer. N Engl J Med [Internet] 2021;(NEJMc2106075). Available from: http://dx.doi.org/10.1056/NEJMc2106075
Plotkin SA, Orenstein W, Offit PA, Edwards KM. Plotkin’s Vaccines. 7th ed. Philadelphia, PA: Elsevier - Health Sciences Division; 2017.
Payne, D. C. et al. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics 125, e438-41 (2010).
Arinaminpathy, N., Lavine, J. S. & Grenfell, B. T. Self-boosting vaccines and their implications for herd immunity. Proc. Natl. Acad. Sci. U. S. A. 109, 20154–20159 (2012).



