Can antibodies from outside of the respiratory tract get in?
A claim has been circulating (pun not intended) on Twitter that current vaccines elicit antibodies that cannot reach the airways, so they can't be protective. That is false.
The gist
There are many ways by which antibodies can get from the blood and into the mucosal surfaces like the respiratory tract. With current COVID-19 vaccines, because they are given by injection into the muscle, they don’t (usually) induce local immune responses in the airways (particularly the upper respiratory tract), which means that antibodies against the spike protein come from the blood (rather than being made directly at the site of infection). As these antibody levels drop, a person might become capable of becoming infected again. This is a motivating factor in pursuing vaccines that can induce this local immunity, such as vaccines given by spray into the nose. Nonetheless, the idea that vaccines don’t work because they don’t induce antibodies that can get into the respiratory tract doesn’t hold water because (1) it’s not true, and (2) we have tons of epidemiological data confirming that the vaccines do indeed work.
The claim
I’ve now seen more than once people claiming that current COVID-19 vaccines cannot generate antibodies within the lungs:
First, my obligatory reminder: you cannot use mechanisms alone to contradict epidemiology. The things that we say are happening at the cellular level should be represented in some way in real-world data for us to care about them. I very much doubt the average person cares about the detailed molecular mechanisms of how vaccines work (if you do though, kudos!)- they care about whether or not a vaccine is protective (and how much). The big picture look at COVID-19 vaccines shows that they are clearly safe, extremely effective against severe disease and death even from Omicron, reduce the risk of long COVID, and reduce transmission (it was hard to pick a single representative study for this so I invite you to peruse this collection of them in Dr. Boonstra’s Twitter thread).
Antibodies in the respiratory tract
Anyway, this claim seems to come from what is ultimately a half-truth: Dr. Haider in the screenshot points out correctly that there is an effort to generate inhaled vaccines, or more broadly speaking, mucosal vaccines (which may be given by routes other than inhalation). The reason for this is because the immune response is somewhat compartmentalized, with the mucosal immune system (that is, the immune system at the mucous membranes like your respiratory tract, digestive tract, eyes, etc.) behaving differently from the systemic immune system (i.e. the immune system on the inside of the body, like the blood). Here’s a cartoon summarizing some of the differences from this review:

Vaccines given by injection into the muscle can be very effective in generating immune responses inside the body, via the systemic immune system, but less so at generating responses locally within the respiratory tract. When we think of antibodies at mucosal surfaces, we generally think of IgA antibodies because at most mucosal surfaces, IgA antibodies (in the form of secretory IgA, sIgA), are the major antibody classes present. However, different mucosal surfaces are different- in particular, in the lower respiratory tract (like the lungs), IgG is actually the major antibody, rather than IgA. In general, we would not expect a vaccine that is given by injection into the arm to produce sIgA in the upper airway, something that has been appreciated while COVID-19 vaccines were still in their development stages and certainly long before that:

How do antibodies get to the mucosal surfaces?
In the figure above, the intramuscular vaccination makes the lungs bright green with IgG1 antibody- so what’s going on? The answer has to do with the confusingly named neonatal Fc receptor (FcRN). The protein was initially discovered as transporting antibodies in the guts of neonatal rats, hence its name, but then we learned it does other things too that are unrelated to that. Fc stands for “fragment crystallizable” and it describes the stalk part of the antibody (as distinct from the pointy tines, called the fragment antigen-binding or Fab region); FcRN recognizes the stalk and is also responsible for actively importing IgG antibodies into the respiratory tract and mucosal surfaces more broadly. FcRN-mediated transport can be used to maintain high levels of IgG at the mucosal surface, so long as blood levels of IgG are also high; as IgG levels decline in the blood, they will also decline at the mucosae, creating the opportunity for infection if other mechanisms cannot compensate.
IgA, in contrast, is transported to mucosal surfaces via a different protein: the polymeric immunoglobulin receptor (pIgR), and cannot bind FcRN.
Having said that, there are lingering open questions about how antibodies end up in the airway in humans. For example, one hypothesis is that antibodies can directly transudate through the airways through gaps between cells. Additionally, there is thought that IgG can enter the upper airway via the gingival crevicular fluid (IgA might also enter through this route but it would not be in the secretory form), and that IgG can reach the upper respiratory tract via the mucociliary escalator after being imported to the lower respiratory tract. Furthermore, beyond all of these mechanisms, one of the major phenomena of inflammation (as would occur if an infection were recognized) is extravasation: the blood vessel cells spread apart to allow the contents of the plasma (like IgG) to enter the site of inflammation, including various white blood cells, causing swelling (edema). Regardless of these mechanisms, what is extremely clear is that antibodies do indeed readily enter the lower respiratory tract at high levels following vaccination.
The Real-World Data
This has been examined numerous times throughout the pandemic, but I’ll highlight a few key examples. First, there’s this assessment of bronchoalveolar lavage (BAL; basically a washout of lung contents, giving you an idea of what’s in the lower respiratory tract) and plasma of COVID-19 vaccinees and COVID-19 patients:
You can clearly see comparable levels of anti-Spike IgG in the BAL between vaccinees and convalescent (hospitalized) patients (this distinction is relevant because hospitalized patients who recover typically produce the highest level of antibodies and mRNA vaccines induce plasma anti-spike IgG levels comparable to those in most vaccinees, which is significantly greater than what is seen in mild infections). However, you will notice differences between the IgA levels in vaccinees vs convalescents (at least for antibodies against the receptor-binding domain, RBD, of the spike protein), with the latter having significantly greater levels, as the 2020 review from Krammer warns would happen.
But, this is the lower respiratory tract alone. For any transmission-reducing effects, we would expect to have antibodies in the upper respiratory tract as well (SARS-CoV-2 doesn’t go straight to the lungs)- and indeed, we do. While plasma spike IgG is high, salivary spike IgG is also high:

Therein is the problem- to keep upper respiratory IgG that would protect from infection high, antibodies in our blood would also have to remain high for that entire duration, which is not presently realistic (although whether this is a limitation of our vaccines, our immune system, or both is not entirely clear).
Additionally, other studies have suggested that the protection from infection itself (not disease) more strongly associates with anti-Spike IgA than anti-Spike IgG, explaining the push in next-generation vaccines to target the upper respiratory tract:
This has also been observed in other analyses.
Some individuals might take these findings and attempt to argue that the best vaccine against COVID-19 is a SARS-CoV-2 infection given that it clearly does induce higher levels of anti-spike IgA, which has been suggested in multiple lines of data to better protect from infection by SARS-CoV-2 than IgG.
As I’ve said before, using SARS-CoV-2 infection to protect against reinfection is comparable to using pregnancy as a form of birth control. There are indeed data suggesting that compared with vaccination, prior infection (given that you survive it), is better at protecting against reinfection than vaccines are against a post-vaccine infection. Nonetheless, these estimates suggest that this protection is dependent on the variant landscape (e.g. if Omicron shows up and you were infected with the ancestral virus, you won’t have the same level of protection as if you had been infected by Omicron), and that this starts to wane significantly after about a year. There are, however, additionally provocative data suggesting that even though reinfection may be less likely in those who recover from COVID-19 than infection is in vaccine recipients, the downstream consequences of that infection may still be better for vaccinated people after correcting for many key confounders.

The possibility of unmeasured confounding here (e.g. differences in testing behavior between vaccinees and non-vaccinees) still exists, but this is consistent with the model that systemic immunity may be more important for controlling disease whereas mucosal immunity may be more important for controlling infection.
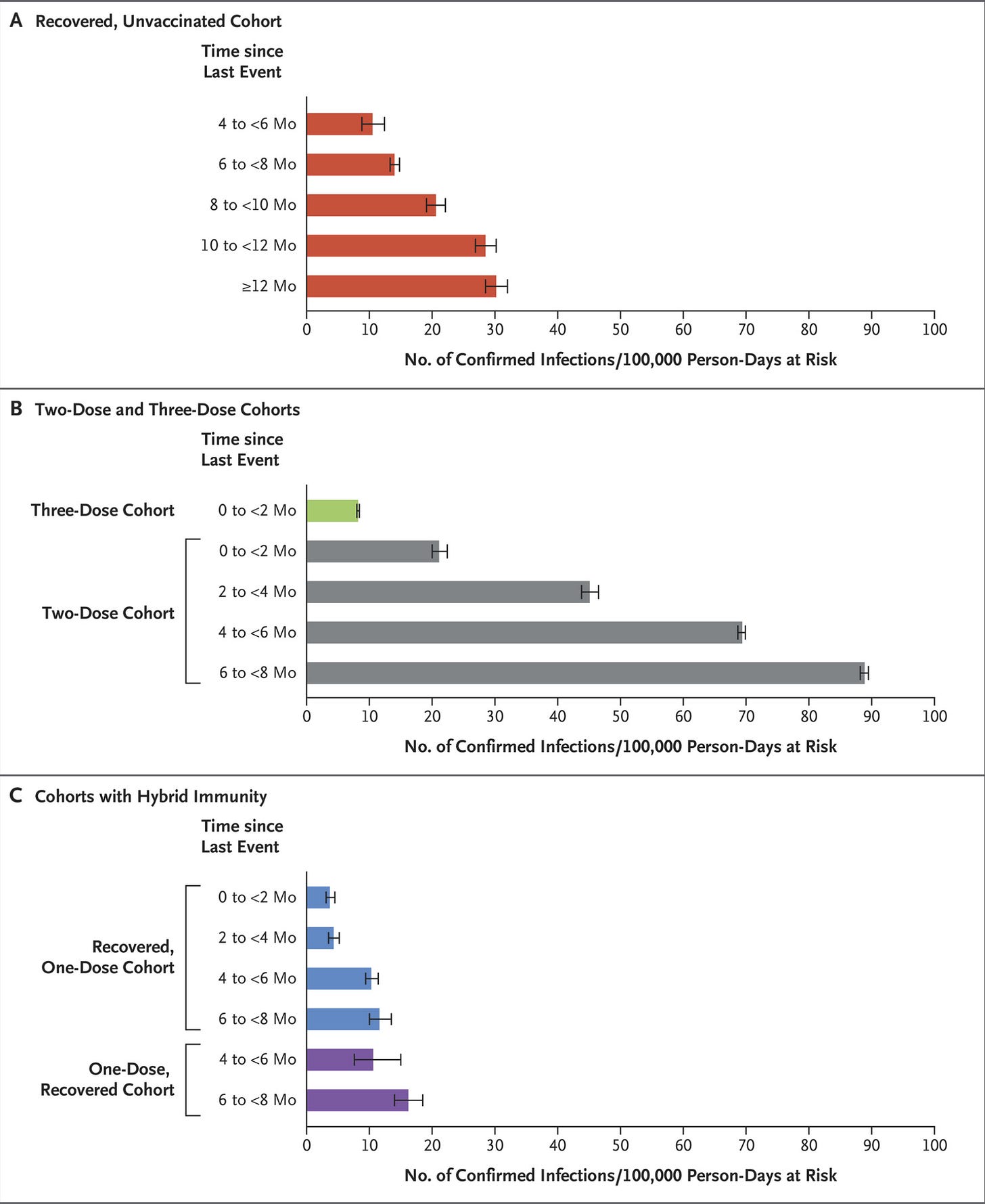
Furthermore, hybrid immunity (immunity acquired following both infection and vaccination) has repeatedly shown to be superior to either vaccine-induced or infection-induced immunity:
It is furthermore worthwhile to note that in previously infected people, secretory IgA (indicative of mucosal immunity) against spike is reliably induced in the saliva upon vaccination.
Vaccination remains important even in those who have recovered from infection with large public health effects (setting aside how critical it is for individual protection in some groups). In the interim, until such time as we have next-generation vaccines that can more durably reduce transmission than our current means, regular (e.g ., annual or biannual) boosters will likely be necessary for at least some of the public for control of COVID-19.