Avian Influenza- Human Perspective
The patterns are concerning, but their meaning as far as human health is not entirely clear.

A brief note: the avian influenza (a highly pathogenic avian influenza, HPAI) discussed here is NOT the same thing as seasonal influenza that causes epidemics in humans every year throughout the world.
The gist:
You, the human reading this who does not work in pandemic preparedness, biosecurity, infectious diseases, etc. do not need to be imminently concerned about the prospect of an avian influenza pandemic in humans. The current situation is undeniably alarming and warrants immediate policy action but the risk posed to humans at the time of writing this appears to be low (but that could change). Nonetheless, you should be aware that this is a problem and it might require some action at some point on your part like getting a vaccine against this HPAI (but hopefully it won’t come to that and I think it probably won’t) and continuing with the precautions you have been taking (or should have been taking) against COVID-19. In the interim, make sure you are up to date on your vaccines and don’t go near dead or sick birds.
What’s happening?
PROMED has been offering updates on it for a while before it started making headlines (if you want to know what’s happening in the world of infectious diseases, strongly encourage subscribing to them), but in short avian influenza (bird flu; specifically influenza A H5N1 clade 2.3.4.4b) has been causing profound ecological damage to bird populations since 2020, with over 60 million dead birds as a result of the outbreak today. 54 countries to date have reported an outbreak of avian influenza beginning in 2021. In 2020, it had spread via migratory birds to Europe, Asia, and Africa. In late 2021, the viruses became prominent in North America, and then gained a foothold in South America by the fall of 2022. More recently, a major scare occurred when it was found that this clade of avian flu managed to infect and spread between minks on a mink farm in Spain, resulting in a peak mortality rate of 4.3%. The mink were eventually culled by November 2022. Spread between seals in New England has also been noted, and even more recently cases among sea lions in Peru have been documented. There have been 6 cases of avian influenza caused by this virus in humans: 4 were asymptomatic or had only mild symptoms, 1 had severe symptoms, and 1 died and as far as I could tell, no further human cases have been reported to date. Risk assessments and situation reports are available from:
UKHSA (a technical briefing from December 2022 is also available)
US CDC (via the IRAT tool) but also a situation report was published in November 2022 with guidance on infection prevention measures
Broadly, they agree that the risk to the general public from avian influenza is relatively low, although enhanced precautions among those in contact with poultry may be needed. Additionally, an adjuvanted human vaccine for avian influenza with the H5 hemagglutinin protein (specifically from influenza A/Astrakhan/3212/2020 (H5N8)- this strain has shown good crossreactivity with the currently circulating H5 hemagglutinin in animals) is on its way to clinical trials with the support of BARDA, GSK, and Sequiris.
Why is this so concerning if the major risk seems to be to animals? How concerned do I, a human, need to be?
Truthfully, I don’t think it benefits the average person to really worry about avian influenza in a personal sense at this stage. I think it’s important for people to be aware that this is a potential public health problem for humans that is being watched and it may require some actions in the future from the general public, but at this time it should not be a cause for personal anxiety (i.e. fear of one’s own self or a loved one contracting avian flu and the downstream consequences of that). Broadly speaking however, this current strain of avian influenza is causing profound ecological devastation through decimation of bird populations and can by extension be devastating for farmers given that the survival of these birds is tied to their livelihood. The finding of infections in a mink farm and spread between seals are particularly concerning because they are mammals and mammal-to-mammal spread could be a herald the possibility of human-to-human spread, which is how influenza pandemics happen. The WHO risk assessment report also notes:
There have been limited reports of transmission between mammals despite the increase in mammalian infections. Affected mammals include badger, black bear, bobcat, coyote, dolphin, ferret, fisher cat, fox, lynx, mink (mink-tomink in Spanish farm), opossum, otter, pig, polecat, porpoise, raccoon, raccoon dogs, seal (seal-to-seal in USA) and skunk.
Avian influenza has also historically been reported to be exceptionally lethal to humans, with a case-fatality ratio (CFR) of 53% using all known cases since 2003 (for comparison, the seasonal influenza viruses we vaccinate against each year and cause devastation to our medical system with a CFR of about 0.1% for bad seasons). Note however that this is based on just 868 cases and we have never observed sustained human-to-human transmission of avian influenza. If avian influenza is in fact this lethal to humans even after acquiring the mutations necessary for it to pass between humans efficiently, the scale of the catastrophe that follows is difficult to overstate. However, that is a big “if” for a few reasons.
Firstly, it is very likely that avian influenza is not nearly as lethal to humans as the CFR would suggest. As this outbreak shows, asymptomatic and mild infections in humans are certainly possible and serosurveys indicate that we have likely significantly underestimated the extent to which this occurs in humans. If these serosurveys are accurate, or at least close to accurate, this measured CFR is likely far off from reality (but still likely more fatal than seasonal influenza and likely COVID-19). On the other hand, some have argued that deaths due to avian influenza in humans are also likely to be under-recognized because of a lack of appropriate tests, and the positive results in serosurveys for avian influenza may not represent true histories of infection by avian influenza because the antibodies induced by seasonal flu strains may crossreact with avian influenza antigens. At the same time, avian influenza has coexisted with humankind for hundreds of years and has yet to adapt for efficient human-to-human spread, raising questions about whether it is even possible for a virus with the severity of avian influenza to also spread efficiently from human to human (and some flu virologists firmly believe it can’t). Note, however, that the more times avian influenzas can infect humans, the more opportunities they will have to adapt to efficient infection of humans (and indeed there are well-defined mutation signatures of adaptation to mammalian hosts seen in avian flu and in the current outbreak). Interestingly, in gain-of-function research using a ferret model, H5N1 viruses that had been adapted to spread in an airborne manner between ferrets lost their lethality. Whether this trend would hold for humans however remains to be determined, and I feel obligated to remind explicitly that none of this should be taken as license to dismiss the seriousness of avian influenza, especially if it does manage to adapt to humans- the possibility that this is our next pandemic is still nonzero.
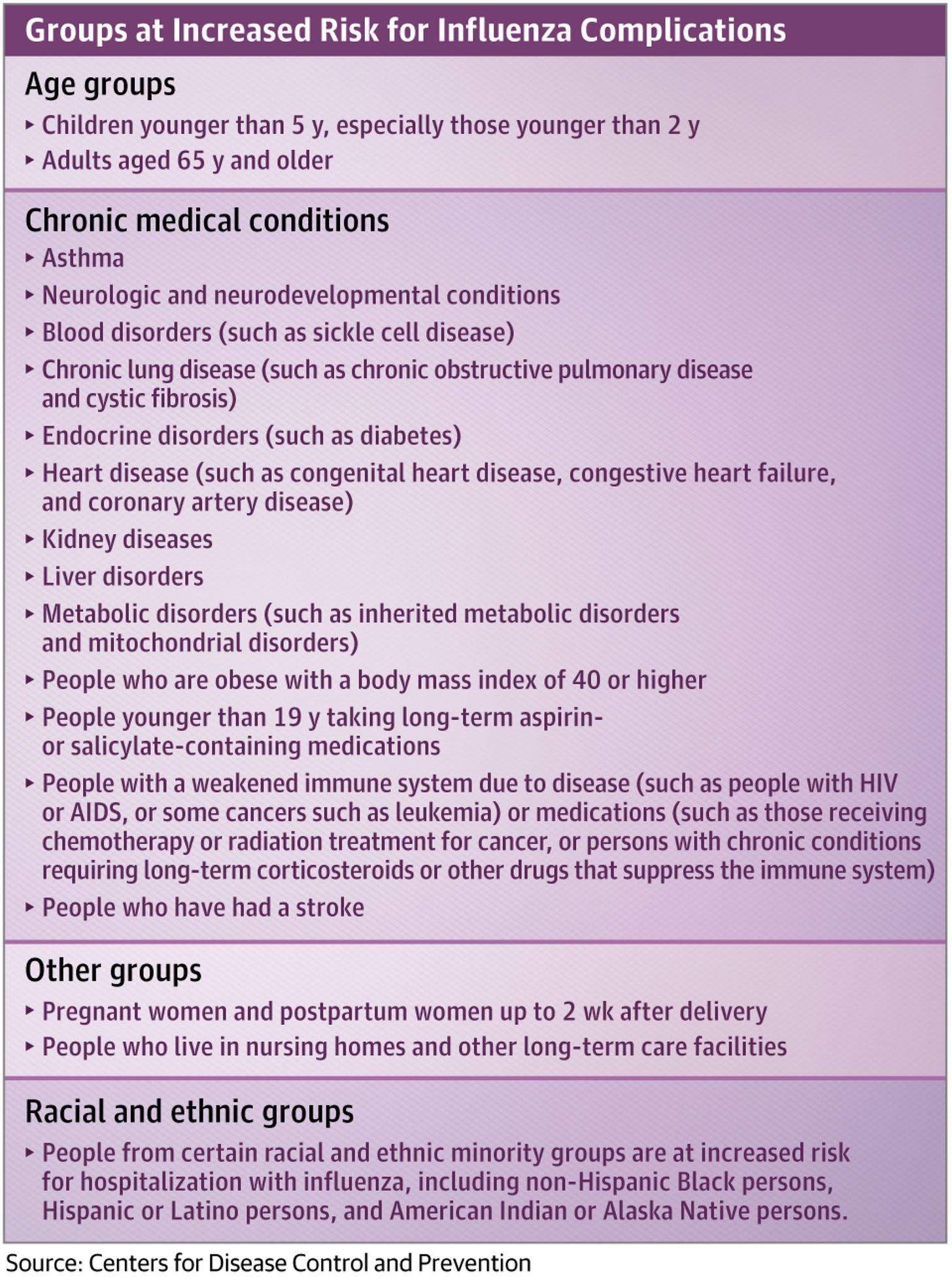
Nevertheless, it would be ideal if we could live in blissful ignorance about what an avian influenza that adapted to humans looked like, and that starts with taking smart precautions against infection. The US CDC offers the summary below:
A lot of this is common sense like “do not handle dead or sick birds.” Note that avian influenza is not just a respiratory illness in birds, but rather is shed in their stool as well and birds may become infected through contact with contaminated surfaces. When humans become infected with HPAIs it is usually because they have had extensive contact with infected poultry or their secretions without the use of appropriate PPE.
Why don’t humans effectively spread HPAIs to other humans?
There have been rare instances of human-to-human transmission of avian flu in the past, but these all generally occurred after extensive contact with an infected patient without appropriate precautions (i.e. PPE) and sustained human-to-human transmission of HPAIs has never been documented. This is commonly rationalized in two ways:

The virus is not able to effectively infect the upper respiratory tract, and thus doesn’t get into the air effectively to infect others.
This is by far the most common explanation you will find, and it sounds compelling- but there are experiments showing that avian influenza can readily infect upper airway epithelial cells from humans in spite of the receptor bias inherent to avian flu viruses. Admittedly, cells in culture, even when taken directly from a person, may not fully recapitulate the conditions in an actual human respiratory tract; however, in autopsy studies of HPAI infections, you can find virus more or less everywhere in some patients, including in the pharynx.
The virus’s replication machinery does not function effectively at low temperatures in the upper respiratory tract.
While there are some data for this, other data shows the replication machinery has no problem working at the temperature ranges in the upper respiratory tract.
So, what’s the actual reason? Unclear. We do know that we still haven’t found good evidence of efficient human-to-human transmission, which is the bottom line. Could it be occurring undetected? Maybe- if human HPAI infections were the sort of illness that could readily be missed (but that raises questions about how dangerous an HPAI infection actually is to people if humans can readily pass them from one another without setting off major epidemiological alarm bells).
What does human infection with avian influenza look like, and how is it managed?
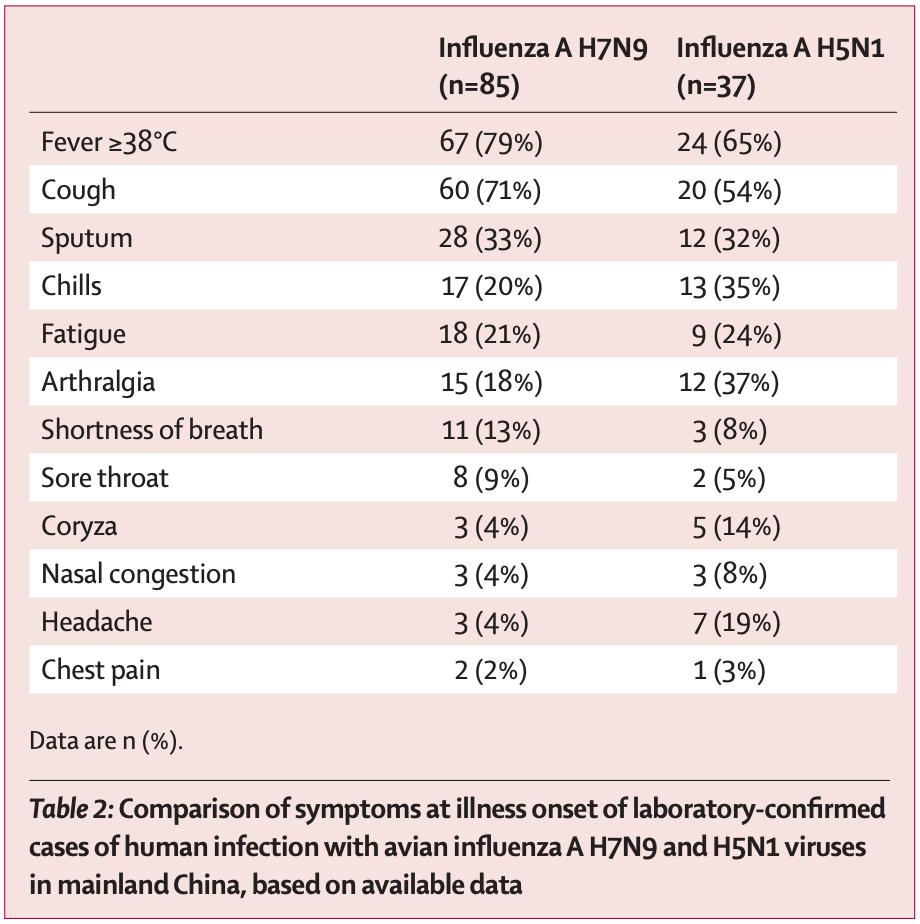
Because of under-detection of cases due to a lack of appropriate diagnostic tools, this question is tough to answer. Avian influenza does not have obvious specific signs or symptoms- it often looks like a bad case of seasonal influenza. At the same time, clinicians should maintain a high index of suspicion for avian influenza if there is a known history of exposure to poultry and should be aware that diagnostic tests will not distinguish between avian influenza and other circulating influenza A viruses. It is thought that one reason that so many HPAI cases in humans are so severe is that they tend to be exposed to huge levels of virus because of extensive handling of poultry (for instance if it is part of their occupation). Fatal HPAI cases can show fulminant viral pneumonia with virus found mainly in the lower respiratory tract but in the trachea as well, and possible dissemination of virus throughout the body. Conjunctivitis is also sometimes seen with HPAI infection and it is thought that the eyes can be a possible route of infection for the virus. More rarely, neurological manifestations such as encephalitis may be seen. HPAI hemagglutinin proteins (which facilitate entry into cells) often contain a polybasic sequence which can be cleaved by the cellular protease furin (this is sometimes known as a furin cleavage site, FCS); furin is present throughout many tissues and so this grants the virus the ability to (in principle) infect a wide variety of cells, partly accounting for the myriad disease manifestations seen. A detailed discussion of possible HPAI clinical manifestations can be found here. A summary of some manifestations of HPAI infections in China is shown in the table below:
Avian influenza has no official medical countermeasures, but the mainstays of therapy are likely similar to those for other forms of influenza. Influenza can be treated with antiviral medications- specifically neuraminidase and endonuclease inhibitors.
To date, the avian influenza in this current epidemic has not shown evolution of mutations needed for resistance to either of these classes of drugs. However, the problem is far from solved. Antiviral medications for acute viral infections are inherently challenging because they have to be given early on to work- ideally within 48 hours of symptom onset or as post-exposure prophylaxis (in the case of oseltamivir). The effects of oseltamivir are not overwhelming in randomized controlled trials, essentially showing a shortening in the duration of symptoms in those who take them therapeutically which should be balanced against the risk of adverse effects from the medications themselves. The endonuclease inhibitors are newer medications, with baloxavir only being available to those over the age of 12 but having the benefit of requiring only a single dose, and shows similar efficacy in RCTs to neuraminidase inhibitors like oseltamivir. The trials for these medications have often been criticized because they were not adequately powered to show improvement in clinically relevant endpoints like the incidence of pneumonia, hospitalization, or death (although the meta-analysis cited earlier did find a benefit for oseltamivir, and observational data suggests a benefit may exist if given early enough, which I think is probably true given what we know about how influenza (usually) causes pneumonia). Influenza, however, is so fast that if the medications are taken after 48 hours of symptom onset (in some studies even after 24 hours), they are unlikely to be effective. At least, this is true for seasonal influenza viruses; for HPAI infections in humans, the kinetics of infection could be quite different and the antiviral medications may be effective for a longer (or shorter) window- we simply do not have that data. In a retrospective analysis of H5N1 cases in children, oseltamivir was associated with substantially lower risk of mortality, which is encouraging, but making firm conclusions about the effectiveness of a medication by uncontrolled, retrospective data is a foolish endeavor. It has also been argued that children do better with HPAI infections because they present earlier for care and have a lower threshold for hospitalization than adults.
Because the window in which antivirals can be protective is so narrow, high-risk individuals who have symptoms, even mild symptoms, suggestive of influenza (see below), should consider contacting their healthcare team about getting tested for influenza and getting antiviral medications if the diagnosis is confirmed. Note that influenza symptoms tend to come on suddenly.
It should be noted however that influenza is capable of evolving resistance to both baloxavir and oseltamivir and other neuraminidase inhibitors (this evolution is theoretically restrained by the fitness costs needed to accomplish this, however).
Aside from antiviral medications, the therapeutic options for influenza are limited. Whereas for COVID we have a number of options with regard to immunomodulators to control the hyperinflammation that occurs in the second week of illness, influenza does not. (High-dose) Corticosteroids are often given for pneumonia due to influenza but in studies this does not show any clear benefit and may actually worsen outcomes, and thus current guidelines suggest they be given ONLY if there is another diagnosis that requires them (more data are needed for low- and moderate-dose corticosteroids). A case-control study also suggested that corticosteroids were associated with increased mortality in avian influenza. Bacterial infections on top of (seasonal) influenza are relatively common and can be managed with appropriate antibiotic therapy but this also does not guarantee a good outcome. Statin use has previously been associated with a good outcome in influenza and other respiratory viruses, but there is no guideline recommending their use (although given how influenza raises the risk of heart attack, I’m inclined to think that offering statins to patients with severe influenza is a good idea regardless but that’s a personal opinion and not medical advice). Hyperimmune intravenous immunoglobulin was evaluated in a very small RCT during the 2009 influenza pandemic and showed a significant benefit but confirmatory data are lacking. Basically, if you get a bad case of influenza, the guidelines do not have concrete recommendations for how to treat you beyond offering supportive care and managing additional problems that arise from it- which is especially appalling given that influenza has been a devastating public health problem for decades and so much research and funding have been spent to understand it. The UKHSA has noted that in those who had known or probable high-risk contact with avian influenza, neuraminidase inhibitors should be given prophylactically.
Because influenza is so difficult to treat, prevention is key, which mainly revolves around vaccination (see next question) and post-exposure prophylaxis in some instances as discussed earlier. In addition to influenza vaccination however, pneumococcal vaccination can be very important in preventing complications of influenza because pneumococcal species can cause bacterial pneumonia in influenza cases, particularly for older adults.
How immunity to influenza and vaccines in humans work
Immunity to influenza is extremely complicated in part because of the incredible diversity of influenza viruses and their ability to mutate. In particular, because the influenza A and B genome is encoded in 8 segments, multiple influenza viruses can infect the same cell and undergo reassortment wherein they exchange segments between the viruses that infected the cell, resulting in a completely new virus with completely different interactions with the immune system than its parent viruses. When this occurs, this is known as an antigenic shift, and this is thought to be the major mechanism by which pandemic influenza viruses arise:

In addition to this, influenza viruses have extremely error prone replication machinery (it does not have proofreading activity) which allows them to steadily accumulate mutations over time that the immune system selects for evasion, a process known as antigenic drift. However, recombination (specifically what is known as copy choice recombination, wherein the replication machinery starts on one RNA and then moves onto another and you end up with a novel RNA encoding part from both template RNAs) seems to be extremely rare with influenza viruses.

Discussions of the immune responses to influenza are often focused on the hemagglutinin protein. Hemagglutinin is the most abundant glycoprotein on the surface of influenza viruses and it is responsible for binding to receptors on host cells (which are sugars called sialic acids, except for the bat influenza virus hemagglutinins which bind to MHC Class II proteins) to allow for infection of influenza and thus antibodies against it can block viral entry into cells (neutralization). There are 18 different hemagglutinin subtypes of influenza A recognized today, grouped into group 1 and group 2 hemagglutinins based on their sequence similarities. Influenza B is divided into Victoria and Yamagata lineages which also differ in their hemagglutinins (there is some thought that the Yamagata lineage may be extinct now because of COVID-19 precautions). The globular head of influenza is the most common target for antibodies against hemagglutinin, but this part of the protein is extremely variable, meaning it is hard for a given antibody against the head to recognize multiple influenza viruses. This has prompted the search for better conserved targets, which has focused efforts on the hemagglutinin stem (or stalk). These antibodies are rarely elicited by either infection or vaccination however, so targeting them remains a challenge. Here the distinction between group 1 and group 2 hemagglutinins becomes immunologically relevant because while it is possible for cross-neutralization within group 1 hemagglutinins through the stalk, there is a sugar present in the stalk of group 2 hemagglutinins that prevents neutralization between hemagglutinin groups (although exceptionally rare antibodies that can bind both group 1 and 2 hemagglutinins have been found). The other downside here though is that antibodies targeting the stalk are less potent than antibodies targeting the head, i.e. it takes more of them to get protective responses. Additionally, the most common way to assess protection from influenza, a serum hemagglutination inhibition titer (HAI- in essence a measure of how well antibodies can block clumping of red blood cells in the presence of influenza virus; a titer of 1:32 to 1:40 is estimated to be where half of people will be protected from influenza, with higher titers corresponding to better protection), cannot detect anti-stalk antibodies. The final problem is that the the reason the hemagglutinin stalk is so well conserved doesn’t seem to be because it is essential in its current structure, but rather because it is not under selection pressure to escape antibodies- when these antibodies are present, the stalk can mutate to escape them. We are quite a ways away from universal flu vaccines (although they are badly needed).

Influenza also has a neuraminidase glycoprotein on its surface (sometimes called sialidase), usually outnumbered about 4 to 1 and harder for antibodies to access because it is shorter. The function of neuraminidase is to cleave sialic acids from the surface of the infected cell to allow for the virus to escape from it (otherwise viral particles end up sticking to the surface of the infected cell and can’t spread to infect more cells), which in effect destroys the influenza virus receptor. Thus, each viral particle has a delicate balance between the number of neuraminidase and hemagglutinin proteins it displays on its surface. Neuraminidase has often been neglected in discussions of the immune response because antibodies directed against it are not conventionally thought of as neutralizing (they can hypothetically prevent new infections of cells by causing virus to get stuck to the surface of the cell it’s trying to bud out of but they cannot prevent an initial infection). Kilbourne however first observed decades ago that responses against neuraminidase are protective and a recent study found that antibody responses to neuraminidase correlated with protection from symptomatic influenza better than responses to hemagglutinin. Examination of neuraminidase as a vaccine antigen has been reignited; indeed it may be present in some currently available flu vaccines. Neuraminidases are also divided into group 1 and group 2 neuraminidases, which are distinguished from one another by the presence of a pocket at a part of the protein called a 150-loop (which is open in group 1 neuraminidases and closed in group 2). With influenza, because there are both neuraminidases and hemagglutinins, the possibility exists that you might have some degree of protection even against a totally novel virus. For example, H5N1 contains a novel (to our immune system) H5 protein, but also contains the N1 protein which always circulates with seasonal influenza (H1N1), opening the possibility that we would have some degree of cross protection. The downside here however is that while the some clinically available influenza vaccines have neuraminidase within them, it is absent from recombinant vaccines, and the quantities it exists in in the rest can be quite low as a result of the manufacturing process and overwhelming focus on hemagglutinin.
Lastly, the surface of the influenza A virion contains the ectodomain of the M2 protein (M2e). M2e is appealing as a vaccine target because it is highly conserved, and preclinical studies have shown some effectiveness for M2e-based vaccines. However, M2e is rare on the surface of the virus and difficult to access by antibodies, so isn’t thought to be the best vaccine target on its own. Current vaccines largely do not emphasize M2e as a target for the immune response.
Underlying these discussions is the idea that antibodies, specifically neutralizing antibodies, are key to protection from influenza. In some sense, this is broadly true- if the virus cannot cause an infection, it cannot cause any of the problems downstream of that infection. However, it has recently been appreciated that there is much more to protection from influenza than neutralizing antibodies. For example, non-neutralizing antibodies can also be protective through engaging other antibody functions. Even more interestingly, a recent analysis (preprint) found that the factor that most strongly predicts protection from symptomatic influenza isn’t actually antibodies, but rather ICOS+ circulating T follicular helper (cTFH) cells (a type of T cell). At the same time, studying the antibody response to influenza has given rise to some important ideas in immunology.
Original antigenic sin
The concept of original antigenic sin comes from observing influenza infections in children. Thomas Francis Jr. first noted that children who had been vaccinated with flu strains they had been exposed to earlier in life made better responses to those strains than to new strains that they had not yet encountered after vaccination, which is what he termed original antigenic sin. The literature on the subject of original antigenic sin is fraught with controversy, inconsistent definitions, and multiple related terms being used such as “imprinting,” “interference,” and “primary addiction” being used interchangeably. The evidence that original antigenic sin is harmful is far from definitive, although it has been proposed that the reason influenza was so dangerous in 1918 specifically to younger adults (giving its infamous W-shaped mortality curve) is that those young adults were primed by infections with an H3N8 influenza virus (which is from a different hemagglutinin group) and thus they could not effectively generate responses against H1. In a recent paper exploring the concept, original antigenic sin is distinguished from imprinting (meaning that your prior immune history shapes your future immune responses; indeed responses to influenza are shaped very much by immune history) by the idea that original antigenic sin is a form of imprinting which suppresses responses against new, related antigens.

Note however that for this to occur requires a certain “sweet spot” of antigenic distance between the original virus and the new one. If the two strains are so different that your antibodies against the original virus don’t recognize anything on the new one, you won’t see original antigenic sin effects, but if there is conservation of some elements that your antibodies can recognize between the two viruses, you may see interference that reduces the extent of the immune response to the new virus (and of course, if you challenge with the exact same virus, your response will consist almost entirely of memory cells from the first encounter). With influenza, the dogma has been essentially that generating broad protection is hard because of original antigenic sin- by which people mean that the memory responses from old infections outcompete new B cell responses and suppress them. However, this probably doesn’t fully capture what’s happening- antibodies that crossreact between influenza viruses also show crossreactivity to self-antigen and experiments suggest that the barrier to these broad responses may be immunologic self-tolerance rather than competition between B cells. From the original antigenic sin concept, some have argued that the secret to broad protection against influenza viruses is simultaneously presenting multiple diverse hemagglutinins to children before they have had a chance to be infected, i.e. antigenic blessings, which is essentially what Francis was suggesting (although it should be noted that after their first influenza infections, children make extremely broad immune responses). This strategy, at least in animals, has shown remarkable success with an mRNA vaccine approach. In humans, the idea of antigenic blessings has been challenged somewhat- limited data from infections in children who had been vaccinated with multiple influenza strains before their infection showed that the influenza infection still had a dominant effect over the vaccines in setting the tone of their immune response (suggesting that current flu vaccines are not good enough at generating immune responses in immunologically inexperienced hosts). Time will tell whether we can create strategies to endow our population with the aforementioned antigenic blessings.
Flu Vaccines and immunity after infection
I will be the first person in line to defend the value of current influenza vaccines- they save lives and are critical elements of our public health response to influenza. The CDC evaluates them via a test-negative design every year and they usually have between 10 and 60% effectiveness against influenza-like illness (ILI), but this doesn’t capture their full effect in terms of preventing mortality and hospitalizations. I would argue that we probably really underestimate how well they work because basically everyone in the population has had flu and that contributes to immune protection, and so flu vaccines are trying to add protection on top of that- which is a really tall order. Nonetheless, better flu vaccines are an unmet need, and in the interim, everyone who can get vaccinated for flu should (detailed US guidelines here).
Broadly, there are two types of influenza vaccine. One is given as a nasal spray and contains live attenuated influenza (meaning that it can infect your cells and replicate to a limited degree but does not cause disease). This concept for the vaccine is thought to more or less mimic the infection and elicits mucosal immunity as well as cell-mediated immunity via killer T cells and IgA. It sounds great in theory but in practice there have been issues. Because the vaccine uses a live attenuated flu virus (adapted to the cold temperatures of the respiratory tract), the more influenza immunity one has, be it from infection or vaccination, the harder it is for the vaccine to stimulate robust immune responses as manifested by more limited ability to replicate. Additionally, seasonal influenza vaccines contain four strains of virus (an H1N1, an H3N2, a B/Yamagata and a B/Victoria), and in a live attenuated vaccine, these four viruses must compete for infections. The downside is you can have a situation where one (or more) of the strains gets really badly outcompeted and you end up with no protection to that corresponding strain of flu. Maybe one day we will have a live attenuated influenza vaccine without these problems— but that day is not today.
The alternative of course is the injected vaccine. This is a whole virus inactivated vaccine but basically ends up being a dose of 4 different hemagglutinin proteins as determined by the strain selection committees (they can contain other proteins but regulators require that the doses of hemagglutinin specifically be standardized by single radial immunodiffusion (SRID), and so other components can be present in variable, even absent, especially in the case of split virion formulations). The injected flu vaccines differ from one another in a few important ways. One is their source. Injected flu vaccines can come from egg culture, cell culture, or be made as recombinant proteins (production methods are described here). Egg-based vaccines are great from a production perspective but… eggs come from birds (chickens in this case) which can present problems (like if suddenly the birds supplying the eggs start dying en masse). There have been instances where the viruses being cultured in the eggs adapted to infection of birds and resulted in a change in the antigens that caused a loss of protection (or at least was thought to). For older adults, because of the changes of the immune system with age, high-dose flu vaccines and as adjuvanted flu vaccines are available. With these flu vaccines, the major purpose immunologically is to give a temporary boost in the antibody response that will prevent infections and reduce spread. These vaccines do not give much of a T cell response (particularly a CD8 T cell response), and their antibody boost is not very long-lived which is why timing the flu vaccines is so important (in the US it is commonly advised to get the vaccines around October/November, just before peak influenza activity; on the other hand, waiting that long may getting infected with flu before you have had any chance to get vaccinated to at least blunt disease severity). It has been suggested that a secondary role for these vaccines immunologically is to replenish influenza-specific T cell populations in the lung which in turn support the antibody response.
There are many next-generation influenza vaccines on the horizon but time will tell how well they perform. For example, mRNA vaccines for flu are currently in trials and would have some major advantages in that they can be updated much quicker than current technologies for flu vaccines to reflect which strains are circulating. However, in early assessments, Moderna’s seasonal flu vaccine did no better in terms of the HAI titer than the Fluzone high dose inactivated vaccine (but HAI titer is honestly not a great metric given how much of the immunogenicity picture it misses, so time will tell whether this holds true in practice). Novavax also has a flu vaccine candidate in trials that uses hemagglutinin proteins and their Matrix M adjuvant. Other approaches seek to fundamentally change the antigen used. For example, chimeric hemagglutinin and headless hemagglutinin vaccines seek to redirect antibody responses to focus on the stalk rather than the head groups. Neuraminidase vaccines and M2e vaccines are also being investigated. Computationally optimized broadly reactive antigen (COBRA) vaccines are another approach which aims to maximally diversify the immune response through specially designed antigens to give as much breadth to the immune response as possible. For live attenuated vaccines, PROTAC vaccine strategies have been what I found personally to be the most exciting, but they may struggle somewhat because of genetic differences across the population in ubiquitinylation machinery.
The other question begged here is: how good is protection from flu after infection? The answer is… complicated. While it’s true that influenza infection by a given flu virus generates very robust immune responses against similar viruses (you can find neutralizing antibodies and memory B cells in survivors of 1918 influenza that happened 90 years earlier- however this should be interpreted with the context that the donors of those antibodies and B cells have been reinfected by H1N1 strains throughout their lives as well as likely vaccinated with H1N1-containing vaccines), they tend to be relatively narrow in their breadth, and human challenge studies show that a year after infection you can be reinfected and develop symptoms with the SAME exact virus. The explanation for this is probably in the fact that influenza attains its peak viral load so quickly that it literally outruns the recall response the immune system generates. This suggests that even with a universal flu vaccine, if we want to really prevent infections (not necessarily just severe disease), we will still probably need boosters on some regular basis unless those universal vaccine achieves unfathomably high levels of antibodies for a very long time (that seems doubtful- the immune system has its limits). Nonetheless, infection with flu does reliably reduce the severity of disease (immunoattenuation) in experimental models. With regard to avian flu, data on the kinetics are much more limited: essentially we know that patients develop very high viral titers when they are very ill but basically nothing about how quickly they achieve that titer, and it’s hard to know how well either infection or vaccination might protect against reinfection (I expect that poultry workers are infected by avian flu strains fairly often but in a clinically inapparent way given serosurveys).
What actions are being taken at the public health/policy level to address the threat of avian flu?
At the moment, most public health agencies agree that this issue is principally confined to animals and the human risk is low; as such the focus seems to be surveillance of the problem in animals. In addition to this, vaccination approaches are being considered in poultry and trials are under way for avian influenza vaccines in poultry workers. When it comes to vaccinating animals, there are several complex issues that make it much less of a no-brainer than vaccinating humans. For one, it is very practically difficult to vaccinate every chicken on a farm, but beyond that, it imposes a serious exposure risk on the part of the vaccinator, and then there are the annoying regulatory and financial aspects. Given the seriousness of avian influenza in humans, the US has a small national stockpile of H5N1 vaccine but spokespeople from ASPR have suggested that it will need to be updated and replenished to reflect the composition of H5N1 clade 2.3.4.4b. Beyond that, we seem to be in the watchful waiting period for now on the human side of things.








Great writeup and appreciate all the sources!