A lot of people may already have antibodies to H5N1- which is really good news. Maybe.
A recent(ish) paper has some optimistic suggestions about the effect of an H5N1 virus circulating in humans- but there are many nuances.
Brief background: For this post it’s important to know that influenza viruses enter cells using the hemagglutinin (HA or simply H) protein and exit cells using the neuraminidase (NA or simply N) protein through the use of sugars on host cells known as sialic acids. Antibodies targeting these proteins can stop viral particles from infecting more cells (known as neutralization), thus protecting us from infections. Their ability to do this can be measured using hemagglutination inhibition (HAI) assays where the virus particles are incubated with red blood cells and antibodies and the ability of the particles to make the red blood cells clump together at different antibody dilutions is measured. In the case of antibodies against N, an NAI assay can be used but it is more complex. For a more detailed background, please see this post.
My good pal Wanderer Jasnah (who you might know for her brilliant and charitable sharing of insights into coronavirology) recently shared a recent(ish) paper with me with a really key finding:
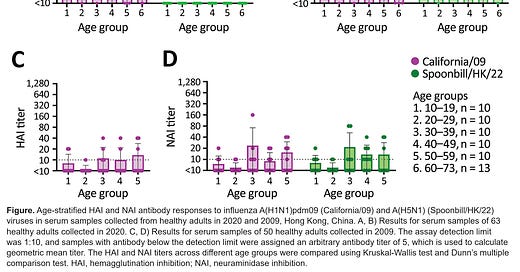
Using a 63 healthy blood donors 18–73 years of age in 2020 in Hong Kong, antibodies were evaluated for their ability to inhibit the hemagglutinin and neuraminidase proteins of the currently circulating H5N1 clade 2.3.4.4b virus (here shown as Spoonbill/HK/22). As can be seen quite readily, there is excellent cross-reactivity between the neuraminidase inhibition (NAI) titers of H5N1 and California/09 (an H1N1 virus) across multiple age groups. Older samples from 2009 (i.e. before/during the H1N1 pandemic) show poorer NAI activity. Unfortunately, HAI titers were undetectable against H5N1.
H1N1 viruses circulate seasonally and have the subtype N1 neuraminidase protein that is also present on the H5N1 strains currently circulating. Neuraminidase is immunosubdominant to hemagglutinin, meaning that antibody responses will tend to target hemagglutinin over neuraminidase (more on that in a bit). However, antibody responses against neuraminidase ARE protective.
On its face, the finding of substantial crossreactivity here (and note that this is not just binding but an assessment of neutralization- the antibodies are functional) is a really reassuring finding. Does this mean we (humans) don’t have to worry about H5N1 as far as our own personal health risk? No- for a few reasons.
Only hemagglutinin (H or HA) content of influenza vaccines is standardized. Depending on which vaccine you get, you can have wildly variable levels of neuraminidase (the recombinant vaccines for example have none). This means that those receiving recombinant vaccines would only have the hemagglutinins from the included strains, which this study suggests, unfortunately, do not generate any meaningful cross-inhibition of H5N11. It has been found previously that current influenza vaccines do not give a substantial response against neuraminidase even though infection does. Nonetheless, some work does suggest that for seasonal influenza, recombinant vaccines may have an advantage over those made in eggs.
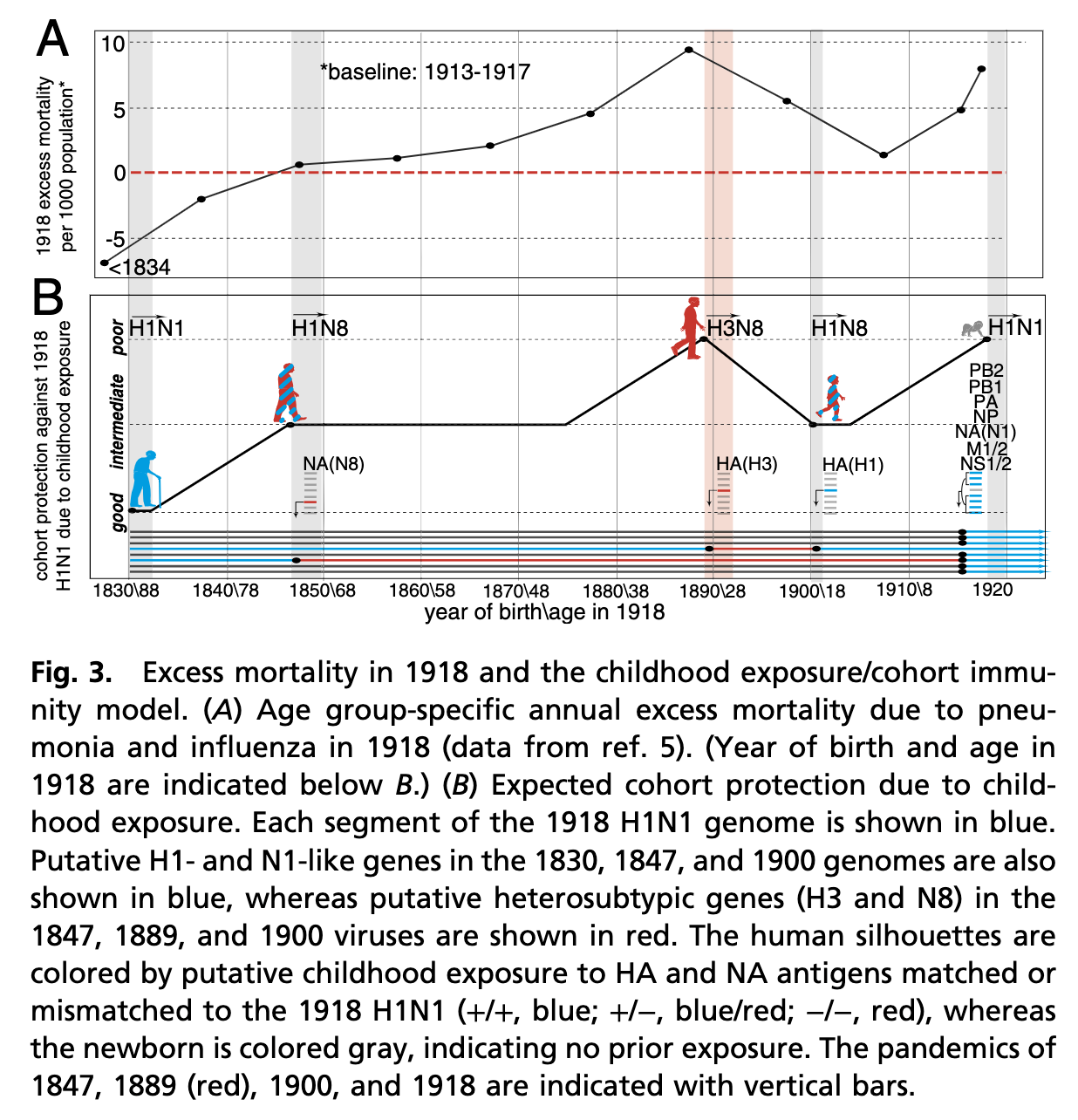
H5 Influenza may not jump to humans in the form of an H5N1 virus. Influenza’s genome is segmented: if two viruses infect the same cell, reassortment may occur. Thus the H5 influenza protein may end up paired with, for example, N8. In fact seroarchaeology suggests that the dominant influenza type from ~1830 to 1875 was an H1N8 virus, which was succeeded by an H3N8 virus, then H1N8, and finally the mother of all pandemics- H1N1:
As this work goes on to suggest, those with early life experience with an N1 neuraminidase or an H1 hemagglutinin fared well against H1N1 because they could work from that pre-existing immunological memory. Some research also suggests that the H5 protein of influenza is a critical determinant of the virus’s virulence (because of its effects on tropism). If avian influenza were to emerge in humans in the form of an H5N8 virus (for example), that could portend grave public health outcomes.
We don’t know what NAI titer corresponds to protection. It’s a frustrating refrain you’ll hear from me but it’s true: antibody titers need appropriate contextualization to be interpreted. Titers can vary widely from assay to assay even when it’s the same sample being tested. An HAI titer of 1:40 (or occasionally 1:32) is generally accepted as being protective in 50% of people (based on studies where participants are experimentally challenged with influenza), but there is no such threshold known for NAI titers, which the authors of the paper do point out. In other words, while this is probably good news, subject to all the previous caveats, we don’t really know HOW good this news is.
We don’t know how well the population as a whole compares to that of the blood donors from this study. A bit obvious, but it bears mentioning- 63 samples of serum from healthy blood donors don’t necessarily inform us regarding the risk of those outside this group e.g. less healthy non-blood donors. For example, a study of Vietnamese residents published in 2021 found some of them had high HAI titers against H5N1 clade 2.3.4.4b. This is mitigated in large part by the fact that H1N1 viruses (the source of these NAI antibodies) circulate seasonally year-round. Put another way, it is reasonable to assume that a very large fraction of the entire world’s human population has significant antibody titers against the N1 protein, though, again, a level corresponding to protection is not known.
What does this mean for the average person?
For now, the situation with H5N1 for the average person is largely what it was when I wrote about it in February of 2023. I might amend it slightly to add that regardless of what’s happening with milk and the infection of cows by H5N1, it is never a good idea to drink raw milk. Pasteurization was not an accident- we should not revel to return to an era where foodborne illnesses were major killers.
As things stand now, we (by which I mean the governments, regulatory bodies, academic scientists, and the pharmaceutical and biotechnology sector) should be working on enhancing surveillance to understand both where this virus is, how it is adapting to its hosts, and aggressively be preparing countermeasures in the event that the worst happens2.
I do feel compelled to point out though that what constitutes “the worst” here is probably not what most people understand it to be when they hear about H5N1. Namely, the number that is quoted for the case-fatality ratio (50% or so) is not an inaccurate number, but it suffers from selection bias. Specifically, serological studies suggest that infections with H5 influenza viruses in humans are much more common than realized and go undetected (some counter with the suggestion that a lack of specific reagents to look for H5 viruses mean that there are fatalities due to this virus that get missed; while this is probably true, the rate of seropositivity in parts of the world suggests that this effect would need to be very substantial for a 50% case-fatality ratio, or something very close to it, to hold). This means that the true fatality ratio of H5 influenza is likely a lot lower than 50%. However, even a small percentage of 8 billion people is still a huge number of lives lost (a lesson we insist on not learning from the COVID pandemic apparently) and death is not the only outcome to think about (another lesson we don’t seem to want to learn).
In short, despite undeniably concerning headlines and unprecedented ecological devastation, H5N1 influenza does not yet show signs of an immediate risk to humans and we do see some lights in this dark tunnel. Nonetheless, it is imperative that we remain prepared for a situation in which this changes. Because it very well might.
Addendum
One really important question that we need to answer is whether or not our seasonal influenza vaccines could potentially offer protection against H5N1 influenza if it were to emerge. One of the key models to answer these questions is the infection of cynomolgus macaques with H5N1 influenza, wherein a protective effect of vaccination with seasonal vaccines could be examined. This has been done by Kanekiyo and colleagues (brought to my attention by corresponding author, Professor Doug Reed). The upshot is: an Addavax-adjuvanted quadrivalent flu vaccine (aQIV) given as 3 doses at 0, 4, and 10 weeks was able to protect against mortality from H5N1 in the monkeys when they were challenged at weeks 18 to 19 post-vaccination:
Unfortunately, its effects on other disease metrics was far more limited. Additionally, consistent with work on human antisera in 2017, these antibodies were substantially lower than those raised against the H1 hemagglutinin, even though both subtypes belong to the group 1 hemagglutinins. Professor Reed has also noted that despite this vaccination schedule, protection against H5N1 seemed to wane very fast:
In short, H5 vaccines may still be needed, and that comes with its own can of worms (perhaps a subject for a future post).
There’s some nuance to be had here too. For one, hemagglutination inhibition titers are not a perfect assay to evaluate the protectiveness of antibodies against hemagglutinin, as discussed in greater detail here. In particular, the stalk of influenza virus hemagglutinin proteins tends to be better conserved, but HAI titers cannot detect these antibodies effectively; indeed, both H1 and H5 are group 1 hemagglutinins. For another, antibodies are not the be-all end-all of protection from influenza. For example, recent work showed a strongly protective effect of T cell subsets against influenza, especially ICOS+ cTFH cells. In the same vein, a nucleoprotein based universal influenza vaccine candidate that relies on CD8 T cells for immunity showed a markedly lower incidence of RT-PCR confirmed influenza among vaccinees relative to placebo recipients in a phase 2a trial (note however that the assumption of proportional hazards is violated as cumulative hazards cross between the groups early on and the study requires confirmation in a large phase 3 trial).
I am personally frustrated by the lack of aggressive efforts at vaccine innovation. The influenza vaccines in routine use today are very similar to the ones that were first invented by Salk and gained wide use in 1945- we have not appropriately harnessed the prodigious profusion of discovery in immunology, structural biology, and vaccinology to get better vaccines to clinic despite the fact that seasonal influenza held the crown for the most devastating respiratory virus for public health before SARS-CoV-2.